It’s that time of year again. The Nobel Committee has announced its choices for the award that recognizes achievements in the fields of Physics, Chemistry and Medicine (Physiology). Since my degree is in physics I think I’ll start with the winners for Physics.
This years winners are being recognized for their work in revealing some of the details about the structure of this Universe in which we live. Three scientists, James Peebles along with Michel Mayor and Didier Queloz will share this year’s prize of 9 million Swedish krona or $910,000 dollars.

Two of the physicists, Professor Michel Mayor of the University of Geneva along with Didier Queloz, who teaches at both the University of Geneva and Cambridge University were honoured for their discovery in 1995 of the first exoplanet orbiting a Sun like star. Today we know about the existence of thousands of exoplanets but it was Mayor and Queloz who used a technique called the Radial Velocity Method to discover an exoplanet orbiting the star 51 Pegasi, in the constellation of Pegasus.
Looking at the illustration below of a star and its planet both orbiting around their mutual center of gravity we see how the star is sometimes moving toward us and sometimes away from us. This tiny tug back and forth due to the gravity of the planet can be seen in a blue shift in the star’s light as it moves toward us and a red shift as it moves away. It was by detecting a repeating pattern of blue and red shifts in the light of the star 51 Pegasi having a period of 4.2 days that allowed Mayor and Queloz to announce their discovery.

The work of James Peebles of Princeton University, the Albert Einstein Professor of Physics no less, deals with a topic a bit bigger and older than a mere planet, the birth of the Universe itself. You see Peebles, working back in the 1970s, was one of the leading scientists who put the Big Bang Theory on a solid theoretical basis.
Doctor Peebles work dealt with probing the Cosmic Microwave Background (CMB) for clues about not only the conditions that prevailed in the Universe at the time of the Big Bang but also in the Universe as it is today. The cosmic Microwave Background is the tiny amount of heat left over from the Big Band that permeates the entire Universe and is almost, almost the same temperature everywhere and in every direction.

It was Doctor Peebles who first predicted that tiny fluctuations in the CMB had to be there. If the CMB was perfectly smooth he reasoned, then the Universe today would also be perfectly smooth, instead of possessing all of the galaxies and stars we see. In other words those tiny variations in temperature 13.8 billion years ago were the seeds from which the structure of today’s Universe grew.
Further analysis of those variations also allowed Peebles to calculate the percentage of the energy of the Universe that today is composed of ordinary matter, the atoms and elementary particles we are familiar with, dark matter and even dark energy which are the subject of so much current research. When you consider how much of our knowledge of the early Universe is due to the work of James Peebles it’s no wonder he has finally received the Nobel Prize.
Since you’re reading this post right now there’s a good chance that you’re using either a smartphone, smartpad, or laptop computer. If so you might want to take a moment to thank the winners of this year’s Nobel Prize in Chemistry. You see the research for which M. Stanley Whittingham, John B Goodenough and Akira Yoshino will share their 7 million krona is the development of the Lithium-Ion batteries that today power our mobile world.

The development took quite a long time and there were more than a few problems along the way to overcome. It began in the 1970s when Stanley Whittingham discovered an energy rich material called titanium disulphide that he used as the cathode, the negative terminal in a battery with a metallic lithium anode as the positive terminal. Whittingham used lithium because of the metal’s ability to release large numbers of electrons.

The problem with these early lithium batteries was that each time the battery was recharged there was an internal buildup of chemicals at each terminal. This buildup would continue until the two terminals actually touched each other inside the battery causing a short circuit that released all of the battery’s energy in seconds. The result of that short would be either a fire or even an explosion. Despite this danger lithium batteries were so powerful that they quickly found some limited applications.
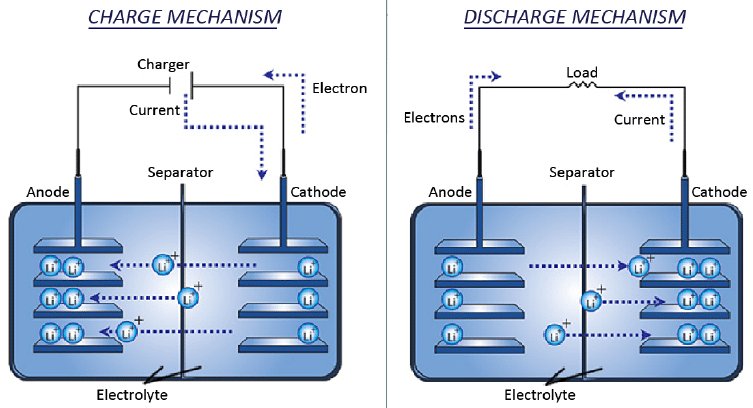
Then in 1980 John B. Goodenough made lithium batteries even more powerful by replacing the disulphide terminal with one composed of cobalt oxide that nearly doubled the energy storage capability. Nevertheless the danger inherent in the lithium battery still kept them from widespread use.
It wasn’t until 1985 that Akira Yoshino succeeded in replacing the metallic lithium with Lithium Cobalt Oxide (LiCoO2) alleviating the buildup of chemicals and making the new lithium ion battery safe enough for widespread use. Thanks to the efforts of these three dedicated scientists the development of the modern lithium ion battery is a case study in how engineering research is carried out, one step at a time. Certainly an achievement worthy of a Nobel Prize.
Also announced this week was the Nobel Prize in Medicine awarded to Doctors William G. Kaelin of Harvard University, Gregg L. Semenza of Johns Hopkins University along with Peter J. Ratcliffe of the Francis Crick Institute and Oxford University. The trio was recognized for their work in understanding how cells adjust their metabolism to match the availability of oxygen.

We are all aware of just how necessary oxygen is for life; the cells of our body will quickly begin to die without that gas. However cells can reduce the amount of oxygen they require whenever oxygen levels become lower. Our bodies experience such reduced oxygen levels during many activities such as swimming or other exercise, or while at high altitude.
More importantly however many people experience low oxygen levels for long periods of time due to lung or heart disease or anemia. In fact the knowledge gained by Doctors Kaelin, Semenza and Ratcliffe is already being put to use to develop drugs that will help patients with those aliments to make better use of the oxygen in their systems and live healthier lives.

The discovery may also be important in the treatment of cancer. You see it has long been known that cancer cells signal other cells in our body to build new blood vessels to them that increases their flow of oxygen enabling the tumors to grow even faster. It is possible that this research may lead to techniques that prevent this increased blood flow thereby slowing the growth of cancerous tumors.
The work of these three Nobel laureates gives our medical science another tool to both fight disease and to understand how living creatures work. Each year the Nobel Prizes are awarded to recognize the best, the most significant discoveries in science. It’s important to remember however that there are many smaller, but still significant advances. All of these discoveries combine to add to our ever increasing knowledge of the natural world.
