With the coronavirus dominating all of the news going on in the world today I thought it might be a good idea today to have a more lighthearted post for a change.
We’re all familiar with coffee rings, the way a bit of spilled coffee or wine will dry to produce a hard, dark line around it’s edge while the center is relatively much paler. See images below.
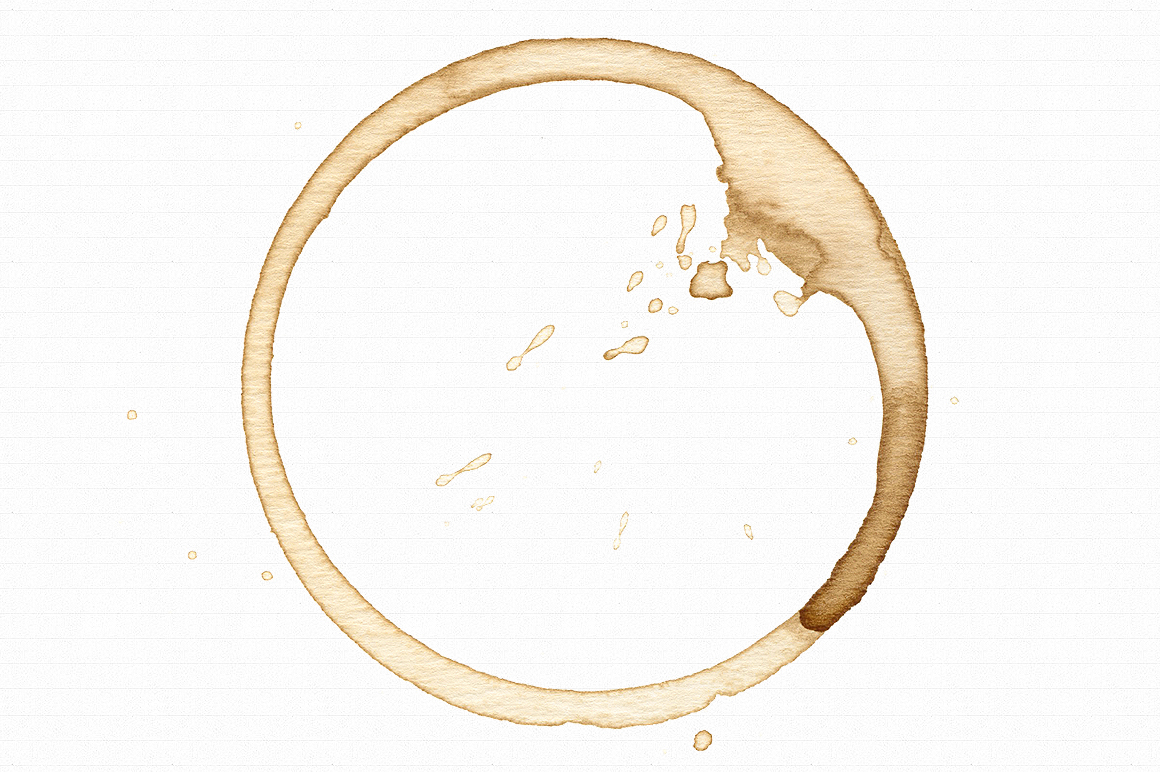
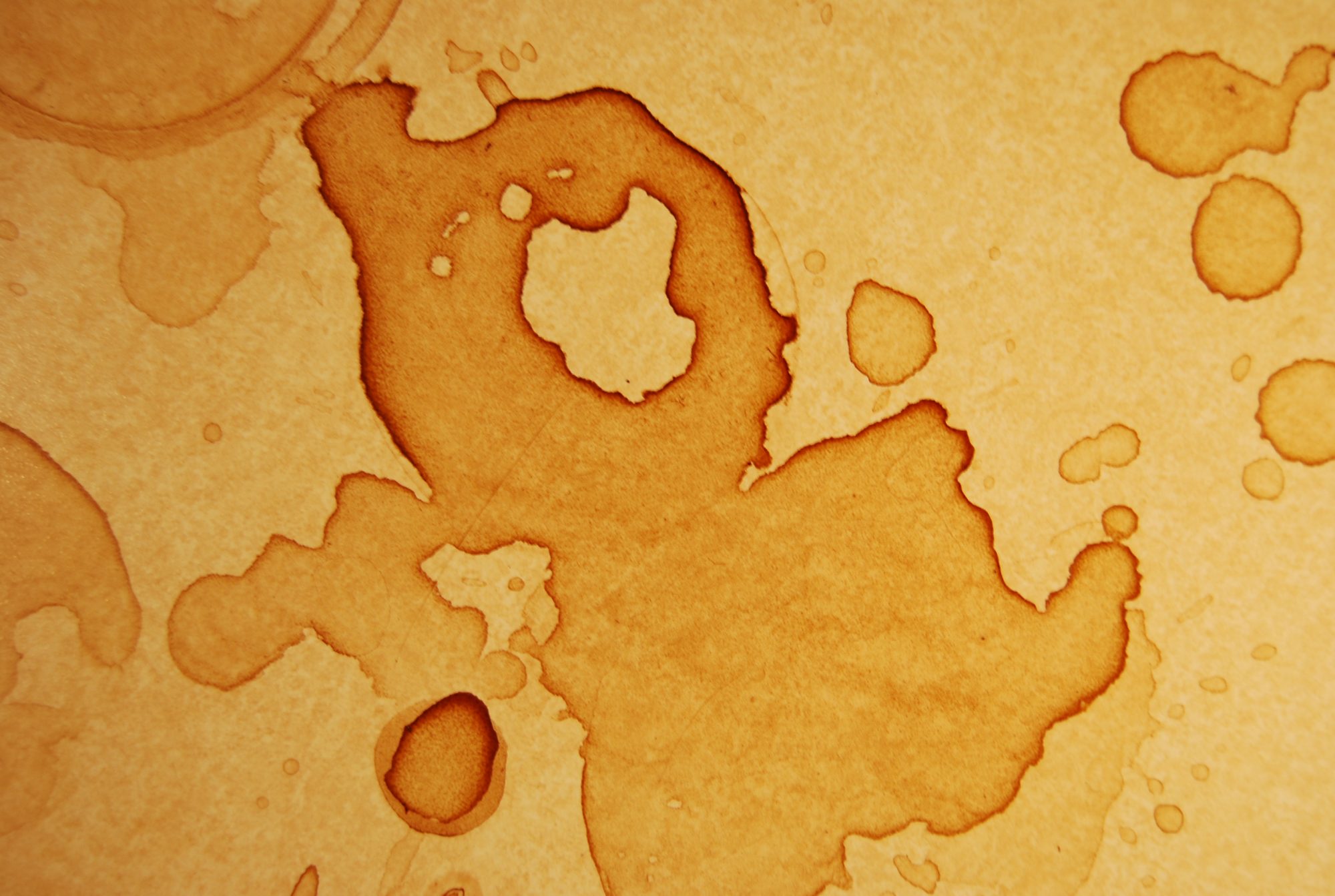
The cause for this phenomenon is a combination of two factors. The first factor is that evaporation of the spilled liquid occurs more rapidly at the edges simply because there is more surface area exposed to the air. Because the edges evaporate faster you would think that the area of a spill would get smaller as it dries but it doesn’t and that’s because of the second factor, capillary action.

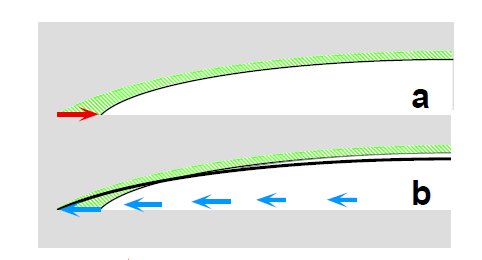
Remember back in your High School Chemistry class when your teacher showed you the way water in a thin glass tube seems to try to climb up the sides of the tube, that was capillary action. Capillary action happens because of the fact that water molecules are more strongly attracted to molecules of other substances than they are to other water molecules. In the case of the glass tube the water molecules are attracted to the glass walls of the tube rather than other water molecules so they literally climb up the walls, forming what is known as a meniscus. See images below.

In a spill, the water molecules are more strongly attracted to the surface they are resting on than other water molecules so even though the edges are evaporating faster the area of the spill remains the same. In order to keep the area the same water flows from the center of the spill to the edges and any larger, darker molecules, like coffee, that are dissolved in the water are carried toward the edges by that flow. As the spill continues to evaporate more and more of the larger molecules are pushed toward the edges to be deposited and left there as the familiar ring once the water is completely gone.
The coffee ring effect can be a problem in some technologies that require an even deposition of a chemical across a surface, such as in printed electronics. Chemists studying the phenomenon have found that adding chemicals known as surfactants, that’s soap to you and me, can reduce the effect.
In an interesting twist on the subject, Stuart Williams, a Professor of Mechanical Engineering at the University of Louisville in Kentucky has been studying the patterns left behind after the evaporation of one of his state’s best known products, Bourbon Whiskey. What Professor Williams has found is that under controlled labouratory conditions instead of forming rings at their edges American whiskeys, and only American Whiskeys, form spider web type patterns. Not only that but each brand of American whiskey has its own unique pattern, a pattern so distinctive that it might be possible to use the technique to identify counterfeit whiskeys. See images below.

Now I said that Professor Williams studied the phenomenon under labouratory conditions and that requires a little explanation because you see alcohol is another chemical that inhibits the formation of coffee rings. Too much alcohol, more than 30% and you’ll only get a uniform coating, no pattern of any kind. At the other end of the scale, too little alcohol and you only get the normal coffee ring effect at the edges. Only when the alcohol content was between 20-25%, or 40 to 50 proof, did Professor Williams obtain his spidery patterns. That means the doctor had to dilute his whiskey samples a bit, a horrible thought but remember this was all in the interest of science!

In his lab Williams tested 66 different brands of American whiskey, 56 bourbons and 10 from outside Kentucky along with 13 foreign whiskeys ( I don’t know how many were from Scotland or Ireland). To date only one of the American brands has failed to produce a web like pattern while none of the foreign brands has formed a web.
As for what could be causing the difference between the American and foreign whiskeys Williams says, “We believe that the increased solids extracted from American whiskeys is responsible for these patterns. The chemicals originate in the fermentation and distillation, but really undergo dramatic changes during maturation.”
Once again each different brand of American forms its own unique spidery pattern. With this in mind Professor Williams is taking numerous samples of each brand and averaging the results in an effort to build up a library of brand patterns. Williams hopes that the whiskey industry will be able to use this library both for quality control as well as spotting counterfeit products.
It’s amusing to think that something as familiar and innocuous as coffee rings may wind up as a way to identify top shelf bourbons. At least I hope it got your mind off of covid-19 for a few minutes!
