Solar energy derived from photovoltaic cells is of course one of the technologies that environmentalists hope will replace fossil fuels as a primary source of power for human society. In order to do that solar cells need to be as efficient as possible in converting the light of the Sun into useful electricity. That’s why for decades now scientists and engineers have worked and struggled to increase the efficiency of photovoltaic materials.
But visible light is not the only kind of electromagnetic (EM) energy; there are others such as radio waves, X-rays and Ultraviolet radiation. One kind of EM energy that could also be gathered as a power source is infrared (IR) radiation, also just known as heat radiation. There are many sources of heat both natural; such as geothermal, and industrial, like furnaces, that could be harnessed for their energy if there were a more efficient technology available.

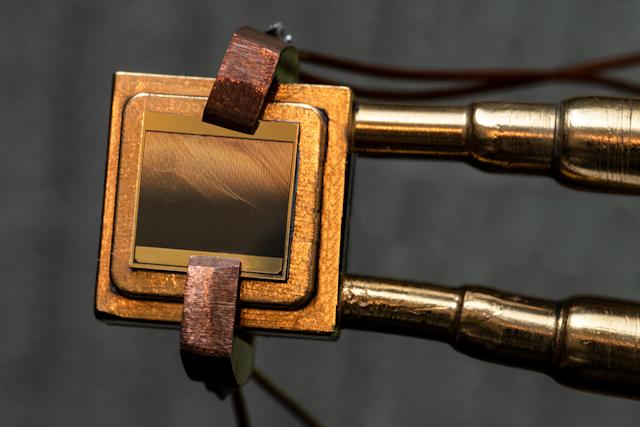
Now there is, for the engineers at the National Renewable Energy Labouratory (NREL) have recently tested a thermophotovoltaic cell that demonstrates a 40% efficiency at converting IR energy into electrical power. That figure is fully 8% better than the previous record of 32% and is actually better than the efficiency of conventional boilers and steam turbines that are currently the most common technology for producing electricity in fossil fuel and nuclear power plants.

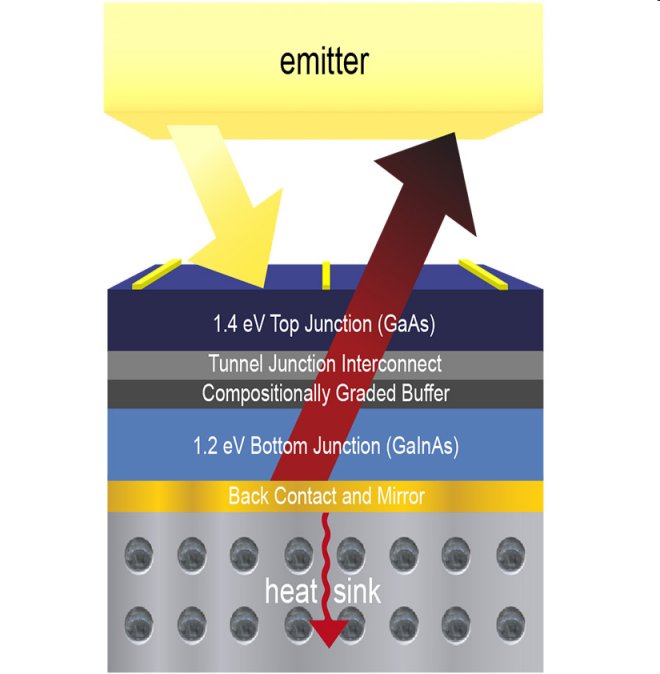
The new type of photocells are manufactured in much the same way that the better known visible photovoltaic cells are except that they possess two light absorbing layers and the entire cell is backed by a reflective layer of gold while sitting on a heat sink to prevent overheating, which decreases efficiency. The version tested is optimized to absorb heat radiation from sources at a temperature of 2,400ºC but that can be adjusted by altering the thickness of the various semi-conductor layers. Thermophotovoltaic devices also have the advantage of not having any moving parts, which both makes them longer lasting while reducing maintenance costs.
The team at NREL hopes that adjustments to the reflective gold layer can increase efficiency further, to perhaps as high as 50%. Nevertheless the development of thermophotovoltaic cells is one more step in our efforts to make better, more efficient use of the energy we already have, one more way of reducing the amounts of CO2 emitting fossils fuels we burn.
Of course the emission of CO2 into the atmosphere is not the only massive source of pollution we humans are currently generating, there’s all of that non-degradable plastic as well. Now in many ways plastics are a miracle of modern science and have improved our lives so much, we mustn’t forget that. They are cheap, can be made in an almost infinite variety of forms, are long lasting and at least initially biologically sterile.

The problem with plastics is that they don’t go away; technically they don’t decay chemically, not for hundreds or thousands of years. And since we use so much of them, and we’re only recycling a small fraction of what we use, they are really starting to pile up everywhere. Also, although they don’t decay chemically they will break down mechanically into smaller and smaller pieces of plastic, pieces that are getting into the biosphere, into the very flesh of plants, fish, birds, mammals and even us!

Because of this scientists have for the last several decades been searching for better ways to recycle or break down plastic into its chemical components so that they can be reused or absorbed back into the environment. Those chemicals that can break down plastics are a special class of enzymes known as polyester-cleaving hydrolases and in 2012 an enzyme called LCC was discovered in Japan that showed some promise as a ‘plastic eater’.
Now chemists at Leipzig University have found a new enzyme that has been found in tests to breakdown a common form of plastic twice as fast as LCC. The researchers, led by Dr. Christian Sonnendecker actually discovered the new enzyme, which they have named PHL7, while investigating the chemical reactions taking place in compost heap in Leipzig itself.

In addition to breaking down plastics faster than LCC, the chemicals that remain after PHL7 has done its work are the exact same chemicals, terephthalic acid and ethylene glycol, from which the plastic was made in the first place, which means the chemicals can then be used to make brand new plastic, a completely closed cycle, the ultimate goal in recycling.

And speaking of plastics we can all do our part in trying to reduce the amount of plastics we use once and then thrown away, plastic drinking straws being one of the most obvious examples. Here in the US something like 200 million plastic straws are used every day, used once and then just tossed away. Each individual straw may seem like a very small thing, a harmless thing but 200 million a day adds up and the results are easy to see anywhere trash accumulates.

Also the type of plastic used for most straws is of a kind that isn’t easy to recycle, and again like all plastics it doesn’t decay in the environment. One way to solve the problem all those straws is to make them out of a material that is biodegradable, a substance that bacteria and other living things can break down and use for food, straws that can be composted and become fertilizer.

Now a new company called Loliware has done just that using seaweed as their basic material. The company, based in California’s Silicon Valley, has developed a process that takes dried seaweed and mills it down. Then, after combining it with minerals and colouring, the mixture is formed into seaweed pellets that can be used in the same machines that are used to produce ordinary plastic utensils. The look and texture of the seaweed utensils are very similar to their plastic counterparts and because much of the same equipment is used in their manufacture the cost is only slightly higher.

So with all of the new, environmentally friendly technology being developed by so many creative scientists and engineers why does it seem as if we’re continually loosing ground in the fight to clean up our planet. Vested interests and simple inertia are the main causes. The oil industry is simply making so much money off of disposable, single use plastics that they can keep prices low, making it hard for biodegradable alternatives to gain a competitive advantage.

Inertia is even more of a problem. We’ve been doing the same things for so long and we just don’t see any reason to change, particularly change to a more expensive substitute. We humans can become so used to the things going on around us that even the massive buildup of CO2 and plastic trash throughout the world we feel is just a part of life, nothing for us to worry about. But the damage we are doing to the only planet we have is real and it’s getting worse all the time. We need for all of us to recognize the danger and if not do something to help then at least get out of the way!
