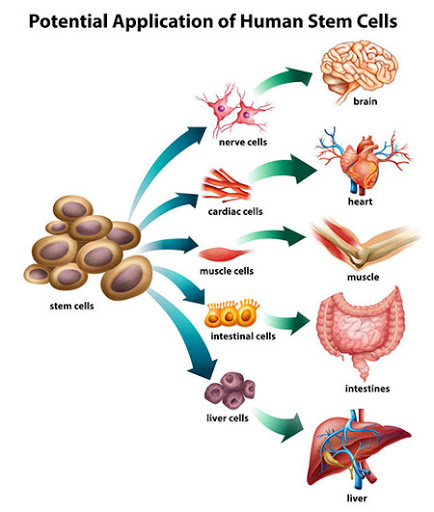
Every human being, indeed every animal begins its life as a fertilized egg cell that begins to divide and grow into many cells. As more and more cells are generated they begin to grow into certain types of cells, heart cells, stomach cells, muscle cells, brain cells, over 200 kinds of specialized cells making up every organ in the body. Those early cells, the cells generated before specialization into organ cells sets in are given the name embryonic stem cells or sometimes just stem cells.
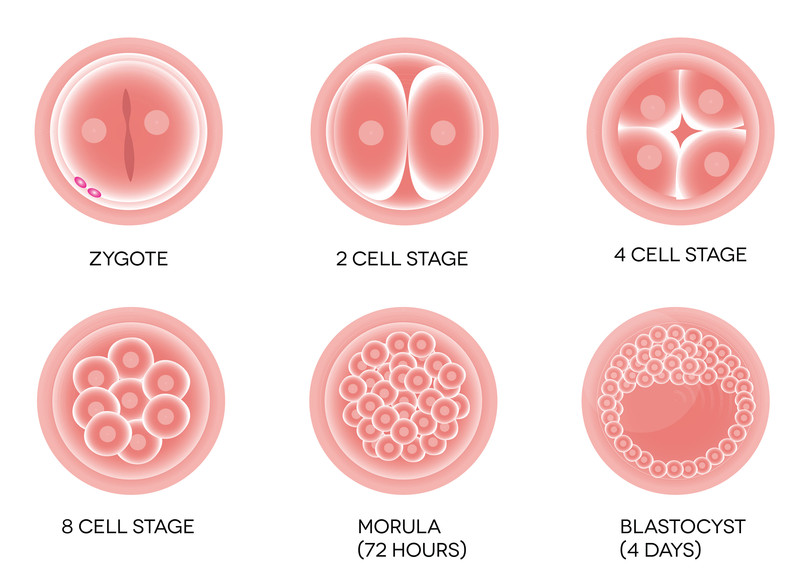
Research into the properties of these undifferentiated stem cells began back in the 1960s at the University of Toronto by biologists Ernest McCulloch and James Till. However it wasn’t until 1981 that British biologists Martin Evans and Matthew Kaufman succeeded in isolating and culturing embryonic stem cells from mice. This advance enabled researchers to begin experimenting with stem cells, to alter or delete some of the genes in the cells in order to investigate the processes that turned them into the specialized cells.

Since stem cells are capable of becoming any type of cell in the body, a property technically referred to as pluripotent, the possibility that they could be used to help repair, perhaps even replace damaged organs has been the driving force in stem cell research. The adult body has few stem cells remaining however, only in the bone marrow or gonads, and those stem cells are only capable of turning into a few types of cells, either blood cells or sex cells.
This is the reason why stem cell researchers were so anxious to obtain embryonic stem cells in order to understand the processes that changed a stem cell into a particular type of body cell. From the 1980s through the early 2000s many biologists conducted an enormous amount of work using embryonic stem cells obtained from animal, primarily mouse fetuses. Unfortunately the only supply of human embryonic stem cells was from the fetuses of women who had undergone surgical abortions, a source that brought with it a tremendous amount of controversy. Because of stem cell research’s association with the practice of abortion even scientists who worked with animal stem cells had difficulties in obtaining funding and the entire field of stem cell research in the U.S. suffered as a result.
At the same time the researchers all knew that in order to really fulfill the promise of stem cells it was going to be necessary for them to find a method to reverse the process, to take differentiated body cells, say blood cells or muscle cells, and turn them back into embryonic stem cells. After all, think about it, if you had a heart problem and doctors tried to use the stem cells from an aborted fetus to repair your heart wouldn’t your immune system reject those stem cells just as it would try to reject a heart transplant. But if your own adult cells could be turned back into stem cells and then those stem cells used to repair diseased heart tissue there would be no problem of rejection.
The breakthrough came in 2006 when a Japanese team led by Shinya Yamanaka succeeded in converting adult fibroblast cells into pluripotent stem cells by modifying only four genes. These converted cells were given the name Induced Pluripotent Stem Cells or iPS Cells and Shinya Yamanaka was awarded the 2012 Nobel Prize in medicine for his achievement.

With the development of iPS cells biologists could now take the adult cells of any individual, convert them into stem cells and culture them into as many stem cells as needed. The focus of stem cell research now shifted from the study of stem cells themselves to learning how to use stem cells to help patients with damaged or diseased organs, a field of research that has become known as ‘regenerative medicine’.
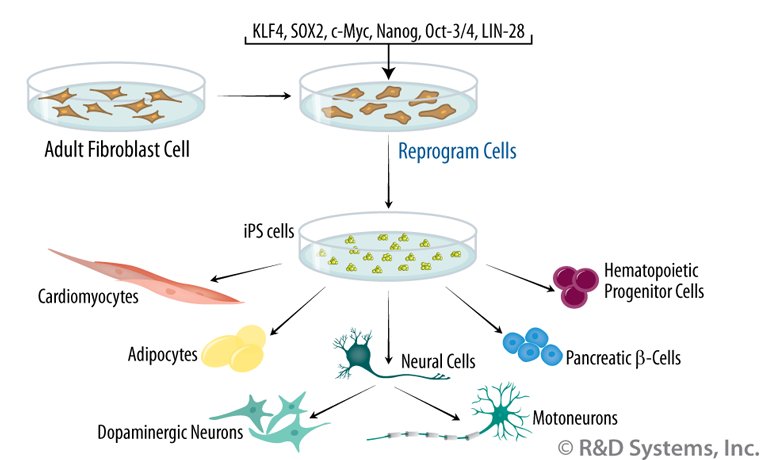
At present there are several distinct lines of ongoing research. The ‘Holy Grail’ of regenerative medicine would be the ‘manufacture’ of entire organs that could replace damaged ones. For example, for a patient suffering from a diseased kidney, instead of getting a kidney transplant from a donor, which would carry with it the problem of organ rejection, cells from the patient’s own body would be converted into iPS cells. Those iPS cells would then be induced to generate a brand new kidney, that patient’s kidney since their cells were used. That new kidney could then be transplanted into the patient’s body without any fear of rejection.
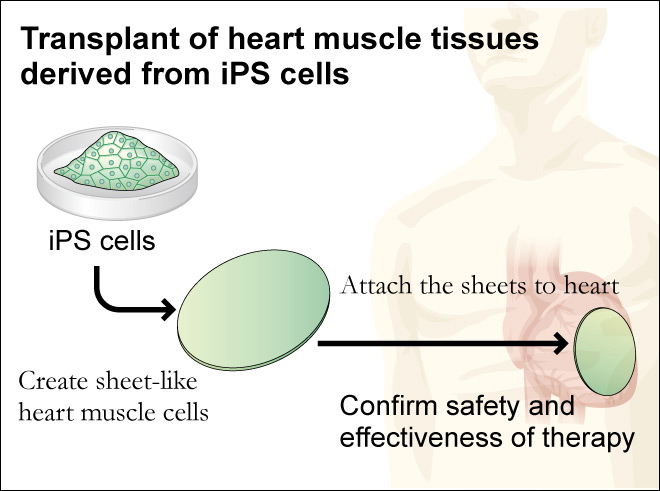
Working towards that long range goal the biologists have been moving forward with the idea of repairing rather than replacing damaged organs. In an ongoing study being conducted at Osaka University in Japan by Professor Yoshiki Sawa blood cells were taken from test animals and converted into iPS cells. The iPS cells were then induced into becoming heart muscle cells that were then grown into a sheet of heart muscle tissue that beated, just like a normal heart. The sheet of heart muscle was then surgically placed onto the test animal’s heart, strengthening it and increasing heart function.
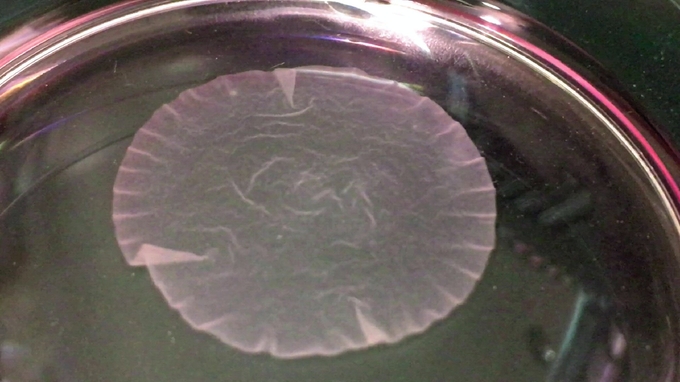
Over a hundred such experimental surgeries were performed first on animals in order to refine the technique and make certain that everything possible was done to maintain safety before any human trials were attempted. It wasn’t until the 27th of January of 2020 that the first surgery was performed to insert a 4cm circular section of manufactured heart tissue on to a damaged area of a human patient’s heart. That patient is recovering and being constantly monitored to determine how much improvement in heart function the new heart tissue is providing, and for how long. Nevertheless this clinical trial gives a little glimpse into the potential of iPS Cells.
Another possible use of iPS cells would be to greatly increase the blood available for operations and other medical practices. Blood banks are chronically short on precious blood plasma so the possibility that that iPS cells could be grown in large quantities and then turned into blood cells is very attractive.
The use of iPS stem cells is not without its problems however. First of all at present the efficiency of converting adult cells into iPS cells is less than 1% making the process both slow and expensive. Another major difficulty is the tendency of iPS cells to form cancerous tumors, a danger that has severely limited the number of human experiments using iPS cells.
Despite these difficulties advances in the use of iPS cells in the field of regenerative medicine is accelerating. Who knows what new medical procedures will be developed in the next 10 to 20 years using iPS cells.

Wow it’s difficult, but it’s a good article. Thank you
A lot of science can seem difficult at first but the more you learn the easier it gets to understand!
Bob L