Arguably the two most well known classes of chemical compounds are acids and bases. Pretty much everybody knows that there is citric acid in our orange juice, carbolic acid in our soda, and of course the anti-acids we take for an upset stomach are actually mild bases. Stronger acids help power our batteries and strong bases like lye make strong soap. So it’s worth asking just what are acids and bases and why is it that they seem to be similar in some ways but complete opposites in others.
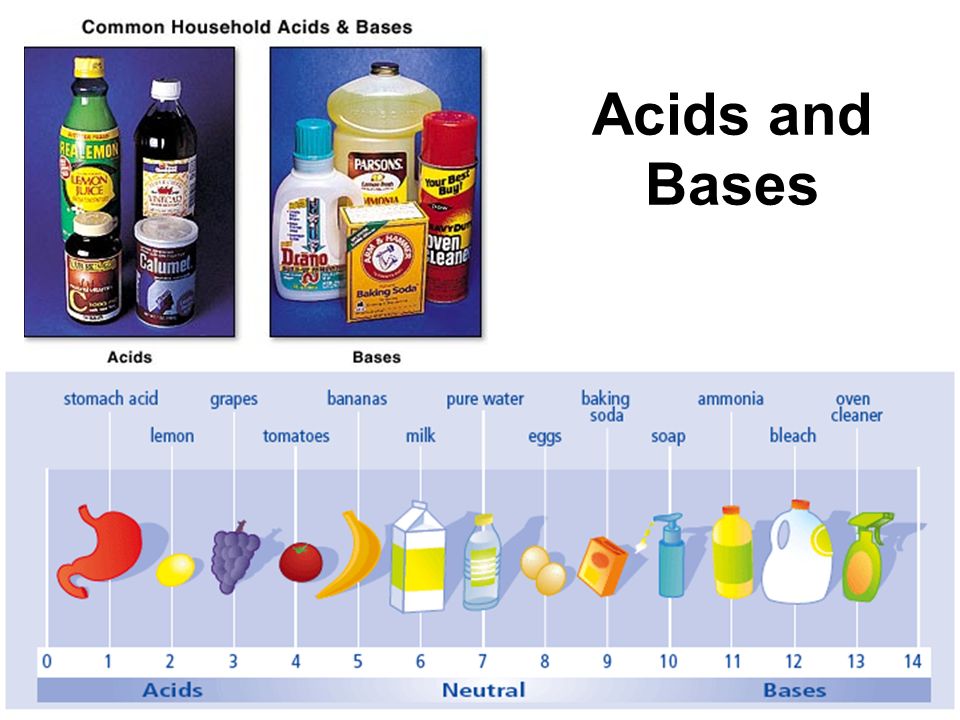
Simply put any element or compound when dissolved in water will form either an acid or a base. When non-metals like sulfur or carbon are dissolved in water they create an acid while when a metal like sodium is dissolved it will form a base. You have to be careful however because you often have to first form an oxide of the element, say carbon dioxide, in order to dissolve it into water.
Since both acids and bases basically form when chemicals are dissolved in water in order to understand them it is first necessary to understand a little bit about water at the molecular level. Now everybody knows that a molecule of water has one oxygen atom and two hydrogen atoms, giving it the familiar chemical formula of H2O. Now all of those trillions of billions of water molecules are moving about in the water, banging into each other so that a few of the molecules get broken up into an OH radical with an extra electron, formally written as OH–, along with a hydrogen atom minus its electron, H+, really just a bare proton.
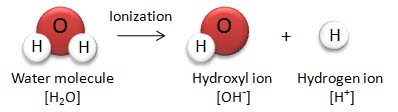
Now these OH– and H+ radicals don’t last for very long, they reform normal water molecules very quickly but new ones are always being made so at any time a constant percentage of the water molecules are split into radicals. At room temperature, 25ºC, just about one in every ten million molecules is split into radicals which when expressed as a power of ten is ten to the minus seventh power, 10-7 and that is why pure, neutral water is said to have a pH of 7! That is what the chemical quantity pH stands for ‘Power of Hydrogen’ or ‘Potential for Hydrogen’ because at a pH of 7 there is about one free hydrogen radical for every ten million, 107 water molecules.
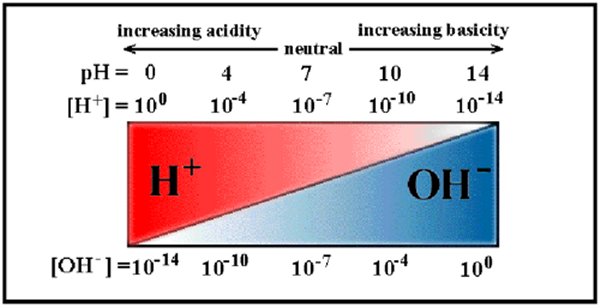
If for some reason there are more free hydrogen radicals, say one for every million water molecules, 106 then the pH goes down to 6. Or if something should cause the number of free hydrogen radicals to go down, say one for every one hundred million water molecules, 108, then the pH goes up to 8. I know this sounds kind of backwards but the more free hydrogen radicals the lower the ph, the fewer the higher the pH.
By the way the pH of even the purest water varies with temperature, water at 0ºC has a higher pH, about 7.47 because at this colder temperature the water molecules are less energetic and don’t split up as often so that there are fewer free hydrogen radicals. On the other hand in hot water the molecules are more energetic and split up more often forming more free hydrogen radicals which causes hot water to have a lower pH of around 6.14.
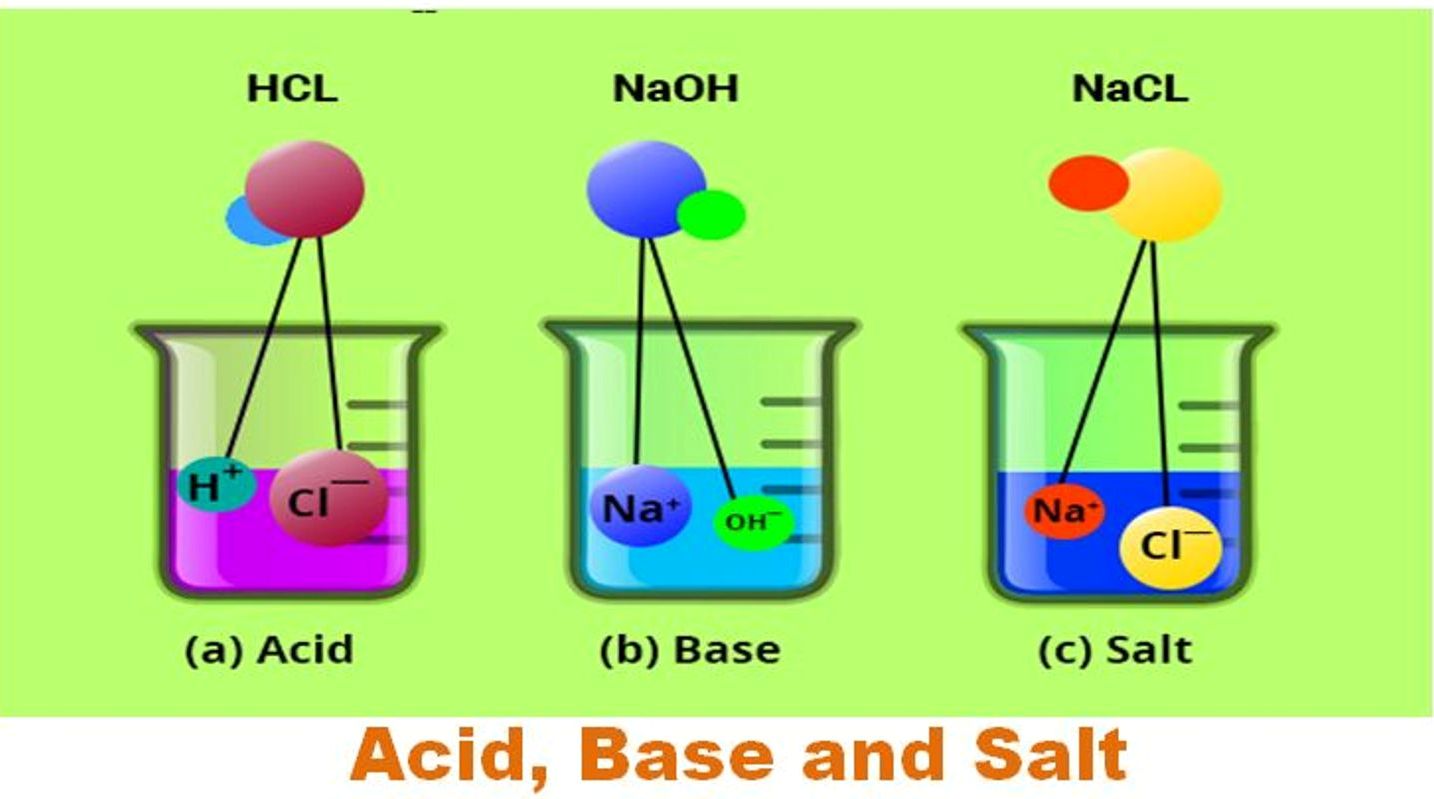
Now let’s see what happens when a chemical is dissolved in water, let’s start with the poisonous non-metallic gas chlorine. Without going into the quantum mechanics suffice it to say that the arrangement of chlorine’s electrons in their orbitals is such that an atom of chlorine needs one more electron in order to fill its shells. When dissolved in water the chlorine atom will grab an electron from a water molecule becoming a negatively charged chlorine ion, Cl–. Meanwhile the water molecule splits into an OH and a free hydrogen radical. The more chlorine the more free hydrogen radicals so chlorine lowers the pH forming Hydrochloric acid. It is the presence of the highly reactive chlorine ions plus the OH and free hydrogen radicals that, depending on the concentration, cause hydrochloric acid to be so reactive, so dangerous.
Metals work in the opposite way. Metals would like to give up an electron in order to have completed electron shells so when a metal, let’s say the dangerous metal sodium, is dissolved in water it gives its spare electron to a water molecule becoming a positively charged sodium ion, Na+. Meanwhile the water molecule now splits into an OH– radical and a neutral hydrogen atom, not a free hydrogen radical. This reduces the number of free hydrogen radicals lowering the pH. Again the presence of highly reactive Na+ and OH– radicals causes sodium hydroxide to be such a reactive, dangerous solution.
The fact is that acids and bases are both highly reactive substances but sort of in the opposite way. So it’s not hard to guess that something interesting should happen when you mix an acid and a base in the proper proportions. Using our two examples above what happens when you mix hydrochloric acid and sodium hydroxide base is that the Cl– ion and the Na+ ion combine to form ordinary table salt NaCl while the remaining OH radicals, the free hydrogen radicals and neutral hydrogen all just recombine into water. This is a typical result, combining an acid and a base in equal quantities results in the formation of a salt and water, plus a lot of energy depending on the concentration.
Because they are so reactive, acids and bases play a crucial role in a huge variety of chemical processes. In fact life itself would be impossible with both acids and bases, the A in DNA stands for acid after all.
Acids and bases are everywhere, in our industry, our food even inside us. It’s worth taking some time in order to know a little bit about them.
