One of the key measures of human progress surely must be a simple counting of the number of different materials that humans have been able to produce and use. Back in the Stone Age, stones were pretty much all our ancestors had to work with, along with wood and some animal bones.

Then during the copper and bronze ages mankind first succeeded in acquiring the skills to use metals, ceramics and textiles. Then came paper, iron and the first use of chemicals such as tannic acid for making leather. The increase in the sheer number of materials used accelerated thereafter so that today we have plastics, semi-conductors, ferrites and petroleum products along with many others.

Glass is one material that we first began to use more than two thousand years ago and today we use it in a thousand different ways. It may come as a surprise therefore to hear that we don’t really know what it is, is it a solid or a liquid! Seriously, when I took inorganic chemistry I learned that glass was an extremely, extremely viscous liquid. You know, something that flowed like a liquid but slowly like honey or molasses only glass is much, much more viscous. In a true solid on the other hand the molecules arrange themselves in a crystalline structure, something that is completely absent in glass.
I’ve seen evidence for this. When I visited the Tower of London in the UK, during the tour I passed by a window whose glass panes were more than 500 years old. Well I could easily see how the glass in those panes was now much thicker at the bottom than they were at the top. The glass was flowing under gravity just like a liquid but it had taken centuries in order to see any visible sign of that flow.

Now describing glass as an extremely viscous liquid may be good enough for a freshman chemistry class. Real chemists however want to understand exactly what is going on, if only because there are a large number of other materials such as proteins, plastics and even some metals that exhibit the same borderline solid-liquid behavior.
A new study by Professors Andreas Zumbusch and Matthias Fuchs at the University of Konstanz and published in the journal Proceedings of the National Academy of Science has revealed new details about the behavior of glass like substances. The researchers carried out their experiments with a class of substances known as colloidal suspensions where very large ‘molecules’, usually formed from long chains of polymer plastics, are suspended in fluids. Now, colloidal suspensions are nothing new to chemists but in most experiments the polymer particles are spherical in shape and about a micrometer in diameter (1μm). What’s different about the experiments at Konstanz is that the polymer particles were especially made with an elongated shape of about 4μm in length by 1μm in width rather than being spherical.
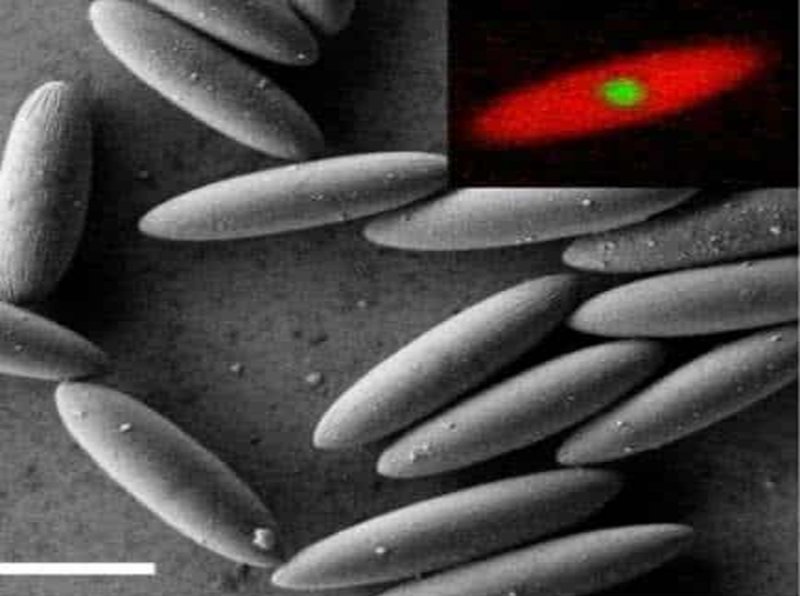
As you might imagine that change in shape of the polymer particles has little effect on the properties of the solution when there are only a very few of them in suspension. In such low densities the solution continues to behave like a liquid, able to flow easily in any direction.
As the researchers increased the density of the polymer particles however the solution soon reached a critical point at which the particles were so close together that while they could still move they could no longer rotate. As Professor Zumbusch put it. “At certain particle densities orientational motion froze whereas translational motion persisted, resulting in glassy states where the particles clustered to form local structures with similar orientation.
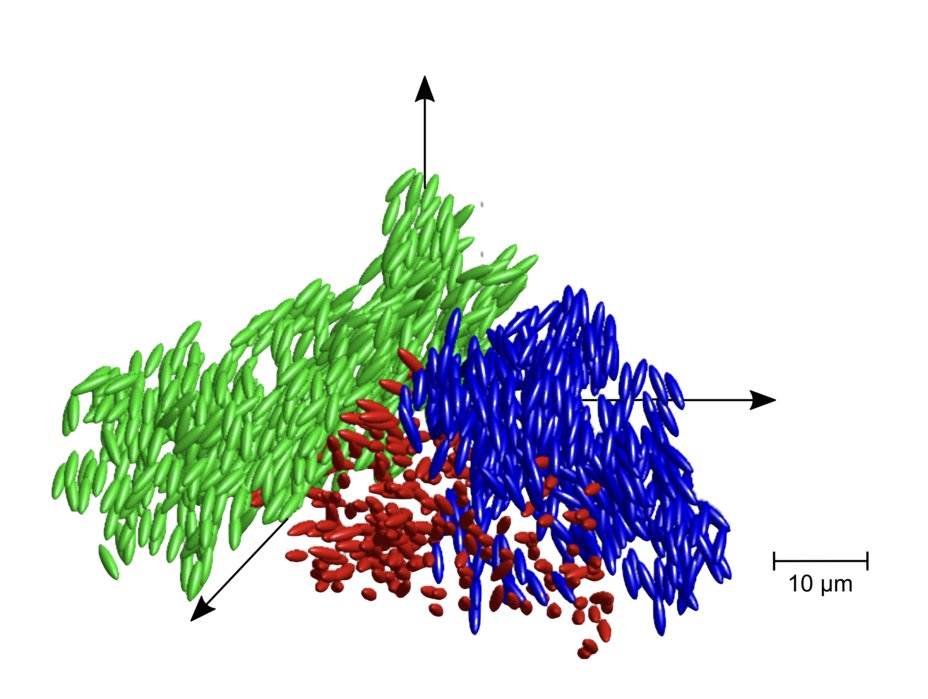
Zumbusch and Fuchs have named their material, actually class of materials, as ‘Liquid Glass’ and are currently busy investigating the properties of Liquid Glass. Their twin goals are to both relate it to more familiar materials such as proteins, plastics and of course glass itself, but also to find more practical, commercial uses.
Human progress has always relied on the discovery and development of new materials. Liquid glass is an entirely new class of materials so it’s strange, fascinating properties hold great promise of future progress.
