We all remember back in our science classes how scientists classified matter into three basic states, solid, liquid and gas and each of these different states have very different physical properties. Solids for example do not change their shape to fit into their container, in fact solids are the only state of matter that holds its shape without a container, and solids are not at all easy to compress into a smaller volume. Liquids on the other hand flow in order to fit their shape to match that of their container but like a solid liquids are not easily compressible. Finally a gas will fill its container like a fluid but unlike the other states it is comparatively easy to compress or expand the volume of a gas.
We were also told that most materials go from one state to another depending on their temperature, water being the classic example with ice, liquid water and steam. Going from a solid to a liquid is of course called melting, the reverse is called freezing while going from a liquid to a gas is either boiling or evapouration, the reverse is called condensation.
Now these classifications are not quite hard and fast, see my post about glass in 23 January 2021. Some materials do have quasi-states where they show characteristics of two or more states at the same time. However, the vast majority of the behaviour of the majority of substances in our everyday lives can be pretty well pigeonholed into solid, liquid or gas.
Or can they? In a paper published in the journal Nature mathematicians at the Skolkovo Institute of Science and Technology in Moscow Russia and the Department of Mathematics at the University of Leicester in the UK have described what they consider to be a new state of ‘active matter’ that displays properties greatly different from those of the other three states of matter.
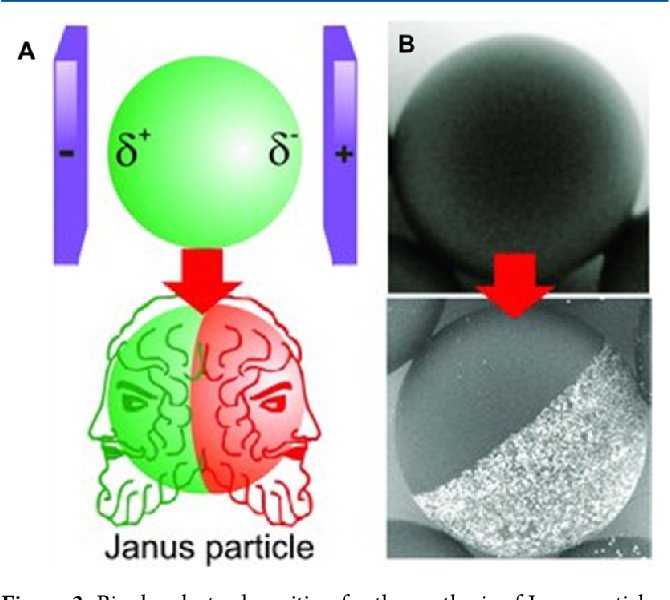
The mathematicians were inspired by the behaviour of schools of fish or flocks of birds but the researchers quickly realized that complex but non-living chemical compounds that react with their surroundings often displayed similar properties. One particular class of complex compounds is known as ‘Janus Particles’ because one side of the particle has different chemical properties than the other.

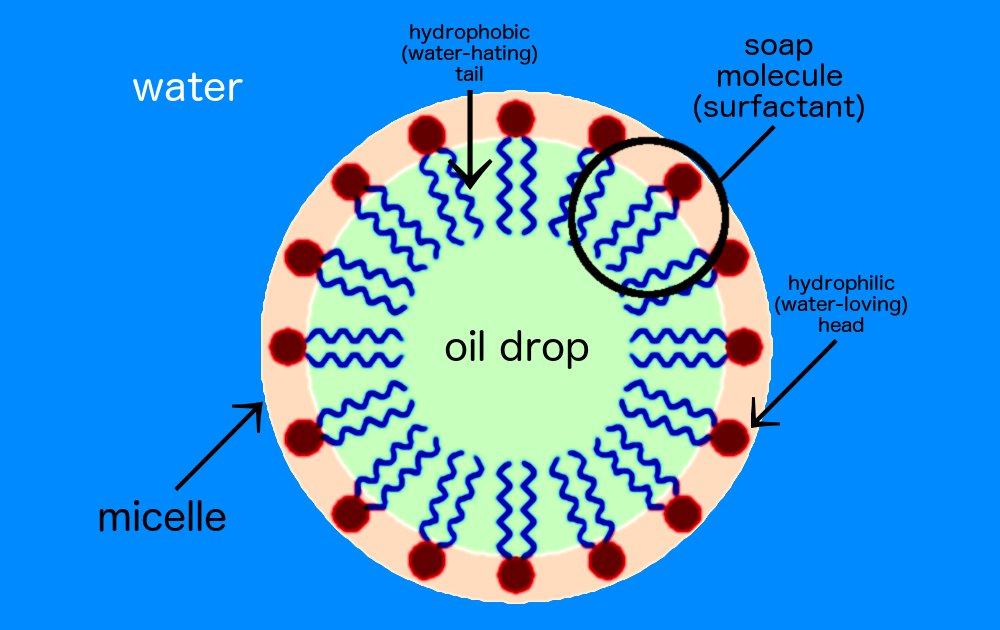
Soap is an excellent example of such a Janus particle, see my post of 2May2020. This is because at one end of a soap molecule is a polar structure that will dissolve in water, a property formally known as hydrophilic. At the other end however is a non-polar structure that will dissolve in oil, a property called hydrophobic. Because of these different properties at different ends when a large number of soap molecules are dissolved in water they tend to group themselves in a circular or spherical shell structures with the polar end facing out and a void in the middle, like a soap bubble. The researchers found that such self-assembly into spherical shell configurations were typical of active matter in general and in addition that the shells showed a tendency to rotate or swirl, which prompted the scientists to name their new state Swirlon.
The mathematicians developed a computer simulation that allowed them to study the behaviour of their swirlonic matter under a variety of different conditions. What they found was that swirlonic matter displayed many strange behaviours including the ability to self-assemble in a variety of shapes.

The mathematicians are now interfacing with Materials scientists who are working with the types of ‘active matter’ they simulated in order to both confirm their results but also to promote new real world materials with new useful properties. In the future the researchers hope to increase the information processing capabilities of their simulated particles in order to better understand the behaviour of living ‘active particles’ like flocks of birds or swarms of insects.

‘Active matter’ is a new class of materials that are now becoming the subject of intensive study, both in the lab and in computer simulations. At the present time we are only beginning to learn how to put their strange properties to useful purposes but several have already shown great promise. New materials with new properties, or in a single word, progress.
