Technically known as the Lanthanoid series of elements the seventeen Rare Earths occupy the upper of the two rows that are always placed at the bottom of a periodic table of the elements. In terms of abundance rare earths are actually not that uncommon, cerium, the most plentiful of the group is actually the 25th most abundant element in Earth’s crust, making it more common than the better known element copper.
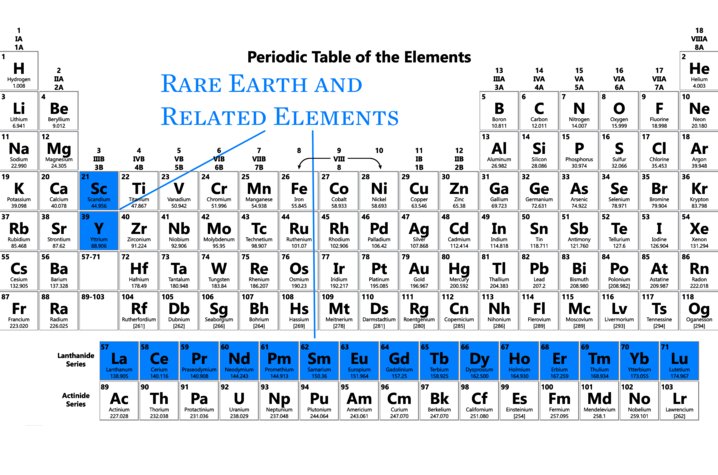
The properties of the rare earths that gave them their name is that in the ground they don’t clump into veins or ores like the more familiar elements do. You’ll never find a nugget of thulium or a rich deposit of gadolinium. Not only that but chemically all of the rare earths behave very similarly. Even if you do find a layer of rock rich in rare earths it is no easy task separating your ytterbium from your neodymium from your erbium.

As you might guess the discovery and understanding of the rare earths was a long and labourious process. The story began with a lieutenant in the Swedish army who was posted to a small village known as Ytterby in Sweden. At a nearby quarry in the year 1787 he identified a new mineral that he named ytterbite but which is now known as gadolinite. That quarry and the surrounding mineral deposits would for the next thirty years be the center of research into rare earth elements which is why so many of them have Swedish sounding names.


Even by the late 19th century when Dmitri Mendeleev was developing his periodic table the rare earths were still confusing chemists. Since the elements didn’t seem to fit in any of columns of his chart Mendeleev put them together in a row of their own at the bottom, and he had no idea how many there were. It wasn’t until the development of X-ray crystallography and quantum mechanics that the relations between the rare earths and other elements, and amongst themselves, were finally sorted out.

And some aspects of the rare earths are still a bit controversial, such as whether they should formally be known as the Lanthanoids, as I have done, or the Lanthanides. Since the suffix -ide is generally used in chemistry to refer to a compound containing oxygen, such as iron oxide or carbon dioxide I prefer to go with Lanthanoid.

Because of the difficulty in processing large quantities of pure rare earth elements their applications in industry and technology were for many years rather limited. Over the last few decades however the uses of rare earth elements has grown dramatically as scientists have studied their unique properties. Perhaps the best known use of rare earths are in co-called ‘rare earth magnets’ using neodymium along with small amounts of terbium and samarium. Other rare earths, such as lanthanum are used in the electrodes of lithium ion batteries while several rare earths, such as holmium and erbium are used in different kinds of lasers. Other current uses include parts for smart phones, digital cameras and computers along with renewable energy technology. As more and more uses for rare earth elements are discovered the demand for them has skyrocketed, as has the price.

In the year’s following World War 2 various countries have held the title of world’s largest producer of rare earth elements with India, Brazil and South Africa each having a turn. During the 1960s through the 1980s the Mountain Pass Rare Earth mine in California was the world’s largest producer. Starting in the late 1980s however China’s Inner Mongolian mines have taking the biggest share of the market and currently manufactures more than 80% of the world’s supply of rare earth elements and, critically nearly all of the heavier rare earths.
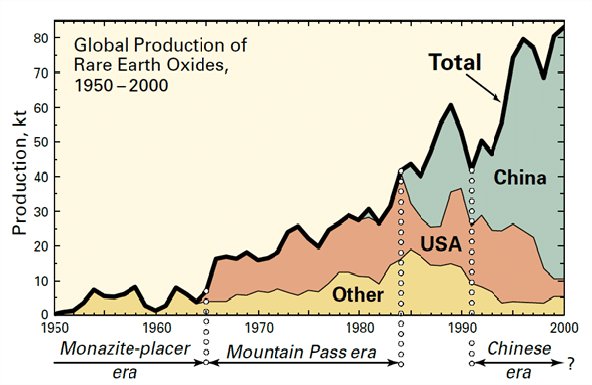
All of which, in these days of political tensions between China and the rest of the world, have made rare earth elements an issue of vital national security concern. Because of this there has been a worldwide search for deposits of rare earths that can be mined profitably. Regions in Australia, Brazil, Greenland and the United States are being explored for possible development.

One further problem in rare earth production is that, since rare earths occur only in low concentration in the best ore deposits, a large amount of earth has to be mined in order to collect any useful amounts. Rare earth mines therefore can be environmental nightmares, ripping up whole ecologies in an effort to procure reasonable quantities of the valuable elements. One possible solution is to mine the leftover, discarded waste from old gold, copper or iron mines where rare earths may be concentrated and the environmental harm has already been done.

Now a new technique has been invented that may be able to increase the quantity and quality of rare earths in a more environmentally friendly way. Researchers at the Pennsylvania State University’s Center for Critical Materials have found that the naturally occurring protein lanmodulin (LanM) will bind to all rare earth elements making it easier to separate them from unprocessed ore.
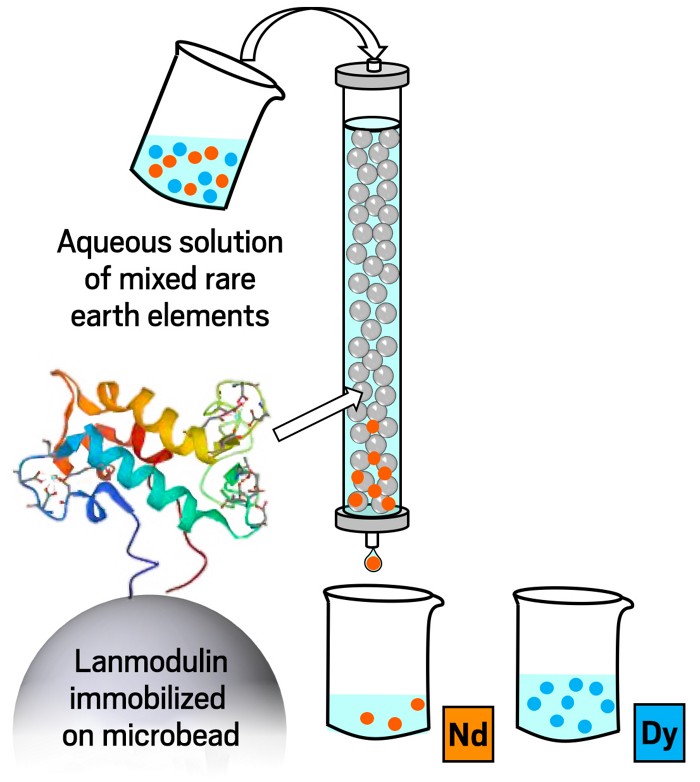
Also, by altering the acidity or adding compounds known as chelators specific rare earths can even be extracted. The researchers are currently working on scaling up the process to industrial levels. If this process can be developed cheaply enough it could be a game changer, converting old abandoned mines into new sources of rare earths. In that case we may just have to stop calling them ‘rare’ earths.
