Over the last two hundred years the science of chemistry has been so successful in developing new energy sources like coal and oil along with new materials like plastics that it has entirely changed the way people live. Seriously just consider all of the materials around your home that did not exist back in the 1820s and you’ll realize just how successful chemistry has been.

But as the old saying goes, “too much of anything isn’t a good thing,” so that today the ‘scientific miracles’ of a few generations ago are now more trouble than they’re worth. The two biggest problems caused by our success with chemistry are undoubtedly climate change caused by the burning of fossil fuels and the enormous amount of trash being generated by single use plastics.


If we’re going to find solutions to these problems then we’re going to need chemists to work on alternative, more sustainable ways of generating power and performing the many tasks to which we put plastic. And in fact such research is being conducted in labouratories around the world this very moment. In this post I’ll discuss some of the results of those efforts.

Sustainable sources of power, such as solar panels or wind turbines are problematic in that while they produce large amounts of energy at certain times, when the Sun is out for solar panels, they produce very little energy at other times, at night for solar panels. The problem therefore is how to store the energy generated at peak production hours so that it can be used when production is low.

One method of storing the energy is to use it to split water molecules into their constituent oxygen and hydrogen atoms. Then the hydrogen produced can be stored in gas cylinders for use in a fuel cell to generate electricity at a later time. Unfortunately the process of breaking down water in the first place requires a catalyst and even with the best-known catalysts the method is very inefficient, wasting large amounts of the energy we’re trying so hard to generate.

Now a team of scientists at UVA and the California Institute of Technology along with the US Department of Energy’s Argonne National Labouratory, Lawrence Berkeley National Labouratory and Brookhaven National Labouratory has developed a unique catalyst that they hope will greatly increase the efficiency of the process. Unlike most catalysts, which are simply salts dissolved in the water, the chemists have developed titanium oxide nanocrystals that contain literally zillions of active catalytic sites on their surfaces. These sites operate at the atomic level to trigger what is known as the oxygen evolution reaction that separates the water molecules into their component gasses.
While the titanium oxide nanocrystals themselves were developed at UVA the Argonne and Lawrence Berkeley Labouratories contributed to the project with the use of their synchrotron X-ray spectroscopy facilities that allowed the chemists to see the catalytic sites in action so that they could more accurately measure their performance.
The physicists at Cal Tech then analyzed those measurements using newly developed quantum mechanical methods. While large-scale implementation is still in the future the results of this team effort have already advanced the drive toward clean, sustainable energy storage.
And new methods are storing power are urgently needed because the installation of solar panels onto homes, businesses and other buildings is now one of the fastest growing industries in the world. And that growth could become even greater if researchers at Incheon National University in Korea are successful in developing transparent solar cells that can double as windows!

In order to develop their transparent solar cell the scientists needed to find two semiconductor materials that are clear in the optical portion of the spectrum but will absorb non-visible wavelengths of light in order to capture their energy. It’s at the junction of the two semiconductors that the absorbed light is converted into electricity.
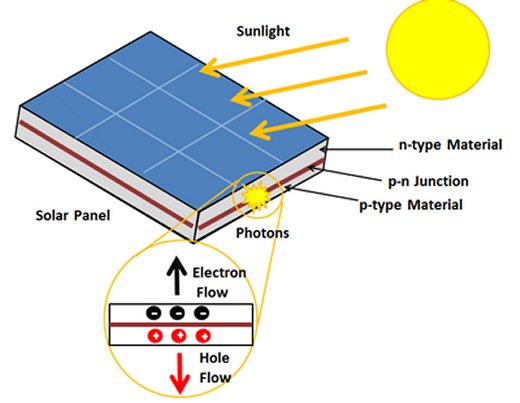
The two materials that the researchers, led by Professor Joondong Kim, settled upon are titanium dioxide (TiO2) already widely used in the manufacture of solar cells and Nickel Oxide (NiO). Both are nearly transparent at visible wavelengths but readily absorb ultraviolet (UV) light. And on top of their optical properties both materials are relatively cheap, non-toxic, and environmentally friendly.
Think about it, in addition to covering the roofs of buildings with solar panels now all of the building’s windows can add their area to producing more energy, and you know the sides of some of those big office are nearly all window! The amount of electricity generated by solar panels could almost double making a real impact in the drive to eliminate fossil fuels.
Replacing fossil fuels with cleaner, more sustainable energy sources may solve one of our environmental troubles but that still leaves the problem of what to do with all of the plastic we keep producing. It’s a real dilemma because plastic is so very useful that we really do need it. When we dispose of it however, it really doesn’t go away. Plastics can take centuries or more to degrade so all the bags, containers, utensils and almost everything you can think of just keeps on piling up until now they are a major environmental threat. What we need therefore is a material that can replace plastic but which is easily biodegradable.
Doctor Antoine Buchard of the Centre for Sustainable and Circular Technologies at the University of Bath in the UK has been investigating that very possibility. Dr. Buchard’s research centers around the sugar xylose that is readily available in wood and is actually the second most common sugar found naturally, in other words it’s cheap!
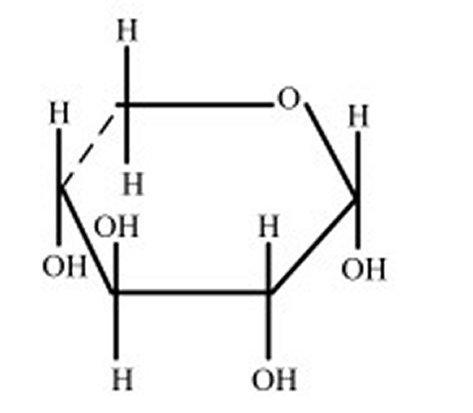
Dr. Buchard has succeeded in using xylose to create long molecular chains similar to those in plastics called polymers. The polymer, which belongs to the family of chemicals known as polyether, can be manufactured as either a flexible or crystalline material. So far the material has shown that it can used to replace both polyurethane and polyethylene but Dr. Buchard hopes to also find entirely new uses for his discovery.
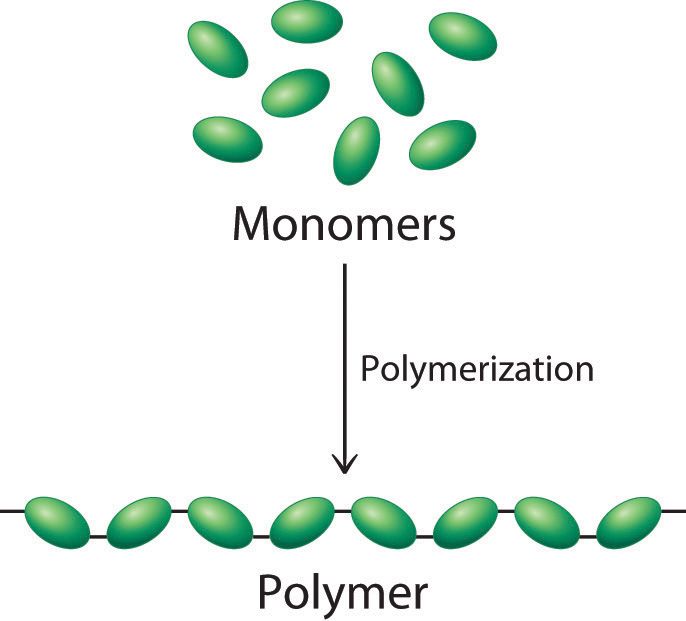
Presently the chemists at Bath are producing the new polymer in small quantities but anticipate that production could be easily scaled up to industrial quantities. Replacing fossil fuel derived polymer plastics with polymers obtained from naturally occurring sugars will go a long way toward reducing the amount of plastic trash that’s choking our planet more and more everyday.
Chemistry got us into these problems by ironically giving us very useful things that we really liked! And chemists are now working hard to find new materials to help solve those very same problems.