The evidence is mounting that the millions of tons of CO2 and other greenhouse gasses that we’re pouring into the Earth’s atmosphere is causing an ever increasing amount of damage to the environment in which we all live. Of course if we’re going to avert the worst consequences of our behaviour the first thing we’re going to have to do is reduce those CO2 emissions as much as possible. The Paris climate accords are the promises that the nations of the World have made to cut back on CO2 but so far few nations are keeping those promises even as the problem grows worse every day.

Let’s be honest, politics being what it is the World’s governments aren’t going to really enforce any CO2 cutbacks until they are forced to by some real disasters occurring, and even then their response will be slow. By the time humanity does finally does start reducing the amount of CO2 we put into the atmosphere there will already be so much up there that the harmful effects on the environment will only continue unless we start taking some of it out.
We need proven, efficient, large scale and most importantly cheap methods for removing CO2 from the air. Because the need is so great you can probably guess that there are many scientists hard at work all over the world on that very problem. Today I’d like to discuss the efforts of just a few.
A lot of the research is taking place at the Massachusetts Institute of Technology (MIT) by Ph.D. candidate Sahag Voskian and his graduate advisor Professor T. Alan Hatton. Together the pair have developed a technique that closely resembles an ordinary battery in construction and application. The device consists of a stack of electrodes that are coated with a chemical compound known as polyanthraquinone that is composed of carbon nanotubes.
It is while the “battery” is being charged that the carbon nanotubes will grab CO2 from an air stream blown across them. Then, as the battery is discharged the CO2 is released by the nanotubes. So what you need is two batteries working in tandem to make the whole system work. One battery will absorb the CO2 as it is being charged, the other does the charging, releasing its CO2 not back into the air but into a containment vessel. Once the two batteries are charged / discharged they can then be flipped, starting the process all over again.

Now the cycle is not 100% efficient, and more energy is usually needed to produce the air flow. Nevertheless Voskian and Hatton estimate that the operating costs will be rather small. Right now the most expensive part of the whole proposition would probably be the carbon nanotubes, which are in fact quite costly to produce. However in large-scale production the costs could come down considerably and remember the carbon nanotubes can be used over and over again so you only have to pay for them once. We’ll just have to wait a see if Voskian and Hatton do have a viable solution for removing some of the CO2 already in our Atmosphere.
Other scientists are taking a more natural approach, developing what they call an ‘artificial leaf’. Led by engineering professor Yimin Wu of the University of Waterloo the team also contains scientists from the California State University, Northridge, and the City University of Hong Kong along with the Argonne National Labouratory in Illinois.
With such an impressive team you’d expect some impressive results and what Professor Wu’s team seems to have delivered. According to Wu. “We call it an artificial leaf because it mimics real leaves and the process of photosynthesis. A leaf produces glucose and oxygen. We produce methanol and oxygen.”
Instead of Chlorophyll the artificial leaf uses a chemical compound called cuprous oxide (Cu2O), which is ground into a powder and mixed into water. When CO2 is blown into the water the cuprous oxide serves as a catalysis separating the carbon and oxygen, releasing the oxygen and producing methanol. The methanol can then be collected and used as a fuel.
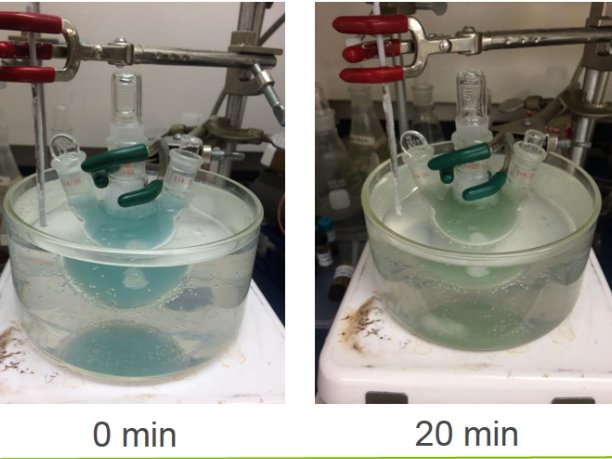
One drawback to the artificial leaf is that, unlike the MIT battery, it requires a high concentration of CO2 in order to work efficiently. This would restrict the usage of the artificial leaf to such places as power plant smoke stacks and car exhausts but once the carbon is collected it is already in a useful form.
There’s still more work to be done. Professor Wu and his team now want to increase the methanol yield while packaging the reaction in a more commercial form, one ready to be used in the fight against the greenhouse gasses causing climate change.
But you know, the artificial leaf got me thinking about the one technology that we could all be using right now to take some of the CO2 out of the air we breath. Real leaves, as in those on real trees. There are now a number of local and world wide organizations promoting the planting of trees as something anyone can do to help fight climate change. In urban locations the trees have the added advantages that they can absorb water during heavy rainfalls to help reduce flooding while even helping to cool entire cities by providing shade instead of just getting hot like concrete and asphalt. So if you want to do you part to help fight greenhouse gasses just take a look around your yard or block. Do you see any places where a nice tree could go?

