After intelligence probably the most astounding ability created by evolution during the history of life is the miracle of flight. The ability to fly gives such an advantage to any living creature that wings have evolved at least four separate times in different animal groups. These groups include the insects, the birds, the mammals and even the reptiles.

The insects were the first to take to the air and fly. We have fossil evidence of flying insects from as far back as the Devonian period when our vertebrate ancestors were just climbing out of the water. Evolutionary biologists have for many years theorized that the first insect ‘proto-wing’ developed not as a organ of flight but instead as an organ to help an insect regulate its body temperature.

You see insects are cold-blooded and on a chilly morning many use the light of the Sun to try to warm up their metabolism. A knob on the insect’s back would increase the amount of the Sun’s heat that the insect could absorb, just like a solar panel, and the bigger the knob the better. Natural selection would then act so as to increase the size of the knobs until they became ‘proto-wings’ that an insect could at first use to glide or even catch the wind for a free ride. As the new wings grew even larger, and acquired muscles that allowed them to move, eventually the insect was able to fly.

Some modern insects still use their wings in that way. If you’ve ever taken a walk in a swampy area early in the morning you can find dragonflies and damselflies climbing up the stalks of tall grasses or reed. Climbing not flying because their metabolism hasn’t warmed up enough to produce the energy needed to fly. Once near the top the dragonfly will spread it’s wings to face the Sun in order to absorb the warmth of the sunlight which will increase its body temperature so that it can fly.
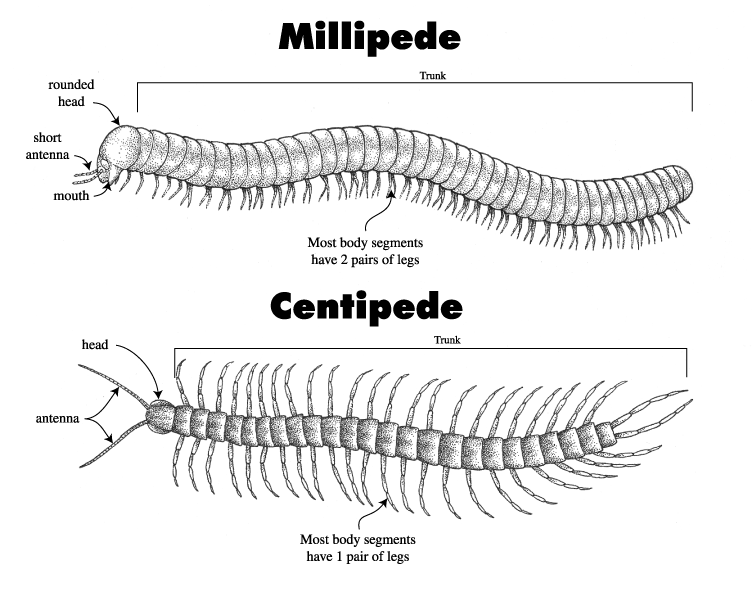
So the question for biologists to answer was, where did those original knobs come from? For over a century entomologists looked in vain among the closest relatives of insects the myriapods, that is the centipedes and millipedes for some structure that could have evolved into those knobs. The search was in vain however because, as modern DNA analysis has shown, insects are actually more closely related to crustaceans, shrimp, lobsters and crabs than they are to centipedes and millipedes.

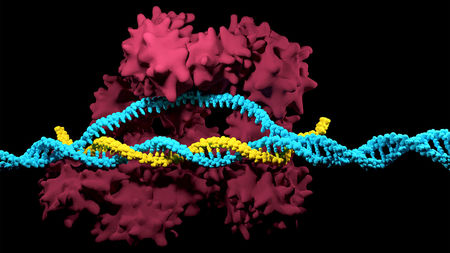
And now a new study from biologists at the Marine Biological Labouratory (MBL) at Woods Hole has discovered the original bump on a shrimp’s leg that developed into the wings of insects. In a paper published in the journal Nature Ecology & Evolution, Research Associate Heather Bruce along with MBL director Nipam Patel have used the gene editing tool CRISPR to demonstrate how a lobe on the seventh, innermost segment of a crustacean’s leg was incorporated into the body of the ancestors of early insects as they moved onto the land. This segment provided extra strength to the exoskeleton of the early insects. In time the lobe then grew to become the long sought after ‘proto-wing’.
Doctor Bruce began by comparing the genetic instructions for the segmented legs of a tiny beach hopper shrimp called Parhyale to those in the fruit fly Drosophila and the beetle Tribolium. Now Parhyale, like all crustaceans have seven segments in their legs while both Drosophila and Tribolium, like all insects have only six segments. However all three species have an identical sequence of five genes that code the instructions for leg development.
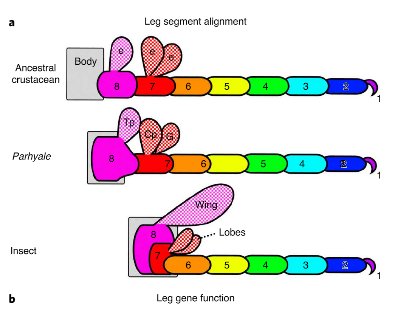
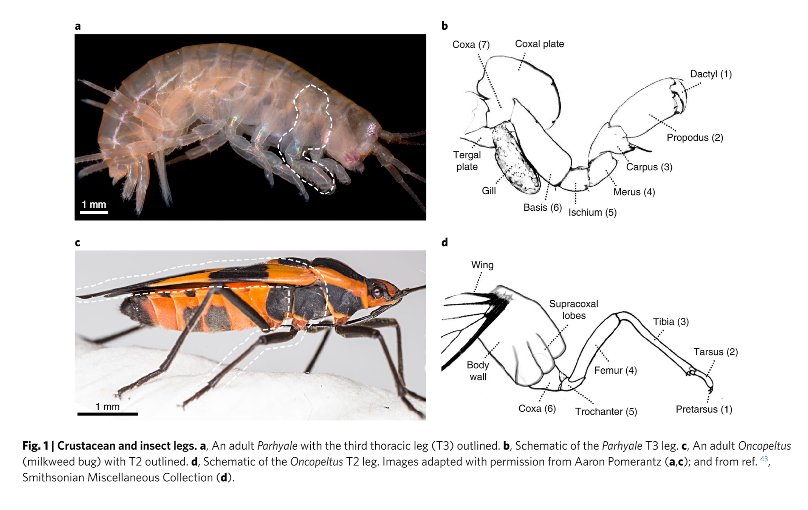
Using CRISPR Bruce disabled those five genes in embryos of all three species one at a time and monitored the results. What she found was that eliminating the genes eliminated the six leg segments farthest from the body. She also found that the seventh, nearest segment of the leg of Parhyale corresponds to a section of the back of the exoskeleton of the insects. Most importantly, a lobe on that seventh segment, called the Tergal Plate moved with the segment becoming a perfect candidate for the knob that evolved into the insect wing. The story of the evolution of the insect’s wing clearly demonstrates the power of natural selection in taking a structure in the body of an animal and altering its shape to perform an entirely new function. The story of how DNA analysis and gene editing have enabled scientists to work out the details of that evolution clearly show the power of the newest tools that biologists possess in their study of life here on Earth.