We all know about diamonds, those sparkly gemstones that are most familiar to us in engagement rings, necklaces and other forms of personal adornment. I’ve actually known someone, a man who had a diamond inserted into a false canine tooth. While rubies, emeralds and sapphires may be valuable its always a diamond that we think of whenever someone mentions a ‘precious gem’.

Another well-known fact about diamonds is that the chemical element that they are made of is ordinary carbon, just like charcoal and graphite. A diamond however can only form naturally when carbon is exposed to the enormous pressure and temperatures that are found deep below the surface of the Earth. Diamond therefore carry with them information about the conditions that exist within our planet’s interior, making them of great importance to geologists.

The difference between a diamond and other types of carbon is due to nothing more than the way the carbon atoms arrange themselves in space. In graphite, the most common form of naturally occurring carbon, the atoms arrange themselves in rings that then form into broad flat sheets. Although the bonds between the atoms in the rings and even between the rings are stronger than the bonds in a diamond the bonds between the sheets are extremely weak. This makes graphite a very soft material.
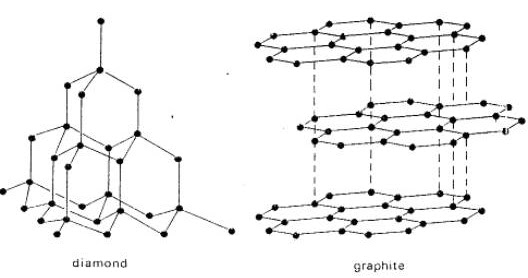
In a diamond however, the carbon atoms arrange themselves in a basic 3-D shape known as a tetrahedron. Each tetrahedron is then connected to adjacent tetrahedrons so that the entire structure repeats itself over and over again in every direction. Because of this 3-D arrangement a diamond is equally strong in all directions.
And we all know that diamonds are the hardest naturally occurring material, a quality that makes them as valuable for industry as they are for jewelry. In fact diamonds are so valuable that material scientists have for the last sixty years been developing techniques with which to manufacture diamonds artificially. Unfortunately, despite all of the heat and pressure modern industry can provide diamond production still requires the addition of a metal catalyst into the carbon that leave the diamonds appearing cloudy, usable in industry but nowhere near gem quality.

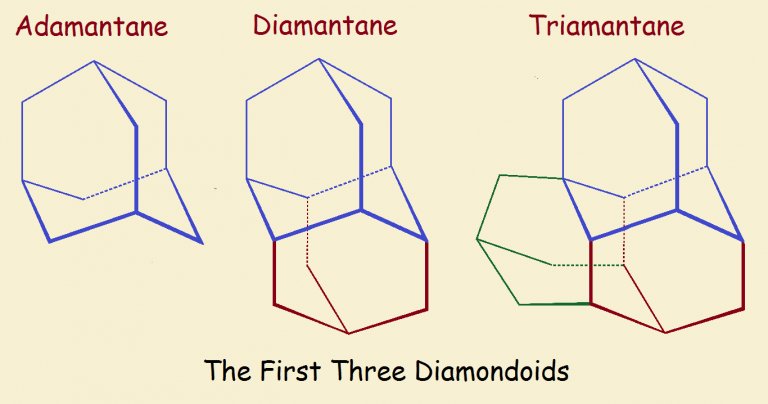
Now a new technique for the production of artificial diamonds has been developed at the Stanford Linear Accelerator Labouratory (SLAC), a technique that requires far less energy and which produces diamonds of gem quality. The key to this new process is in the raw material, a white powdery residue that is left behind in empty containers of crude oil. The researchers, led by senior author Yu Lin, recognized that the grains of the powder were in fact 1-cage to 3-cage carbon ‘diamondoids’, small fragments of diamond with atoms of the element hydrogen filling in around the edges.
The researchers took the fine grains of ‘diamondoid’ and placed them in a pressure chamber; actual diamonds are used as the chamber’s inner walls. At a pressure of 20 gigapascals, similar to that deep within the Earth’s interior, the ‘diamondoids’ are zapped with a laser to a temperature of 900 Kelvin, just a fraction of the 5000 Kelvin required for natural diamonds. The ‘diamondoids’ fuse together, eliminating the hydrogen in the process and the result is a tiny pure diamond, gem quality although much too small to be used in jewelry.
Presently the process developed at Stanford is impractical to be employed on an industrial basis, but remember it’s brand new. Who knows what improvements could be made over the next decade or so. Also the Stanford technique has taught Doctor Lin and her colleagues a great deal about how diamonds form, knowledge that could spur those improvements and lead to a better understanding of these precious gems.
Before I close I’d like to take a few minutes to discuss the diamond industry itself. First of all diamonds really aren’t all that rare, certainly not rare enough to justify the high cost of diamond jewelry. In order to keep prices high however the diamond merchants employ a great deal of advertising to convince people that a diamond is some sort of a visible, material symbol of love.

Another trick played by the diamond industry is that fully half of the gem quality diamonds that are brought out of the ground are simply locked away in storage vaults. In this way, by deliberately restricting the supply the price of diamonds in jewelry is kept artificially inflated and consumers are convinced they are buying something much more rare and valuable than it really is.
Additionally of course there is the problem of conflict diamonds. It’s true that many of the gem producing areas of the world are also war zones and both dictators and rebel leaders have used the sale of diamonds and other gems to help finance their murderous careers.

All the more reason therefore to hope that the researchers at Stanford will succeed in developing a method to manufacture gem quality diamonds on an industrial scale in order to reduce the value of diamonds. Maybe then diamonds will no longer be used as weapons of war or con-games to bilk innocent consumers.
