In this era of Covid-19 we hear one piece of advice dozens of times everyday, ‘Wash Your Hands’, ‘Work up a good lather of Soap and Warm Water and Wash your Hands while singing Happy Birthday Twice!’ Which begs the question, what is Soap? Why is Soap so central to both cleanliness and good hygiene?

Chemically soaps are a class of compounds known as salts of fatty acids and are produced by combining fats or oils with an alkaline base in solution under heat, a process technically known as Saponification. Soaps include a wide range of substances used for a variety of purposes from thickening agent to lubrication but the most familiar use of soap is undoubtedly as a cleaning agent and in this post I will mainly be referring to these types of soaps.
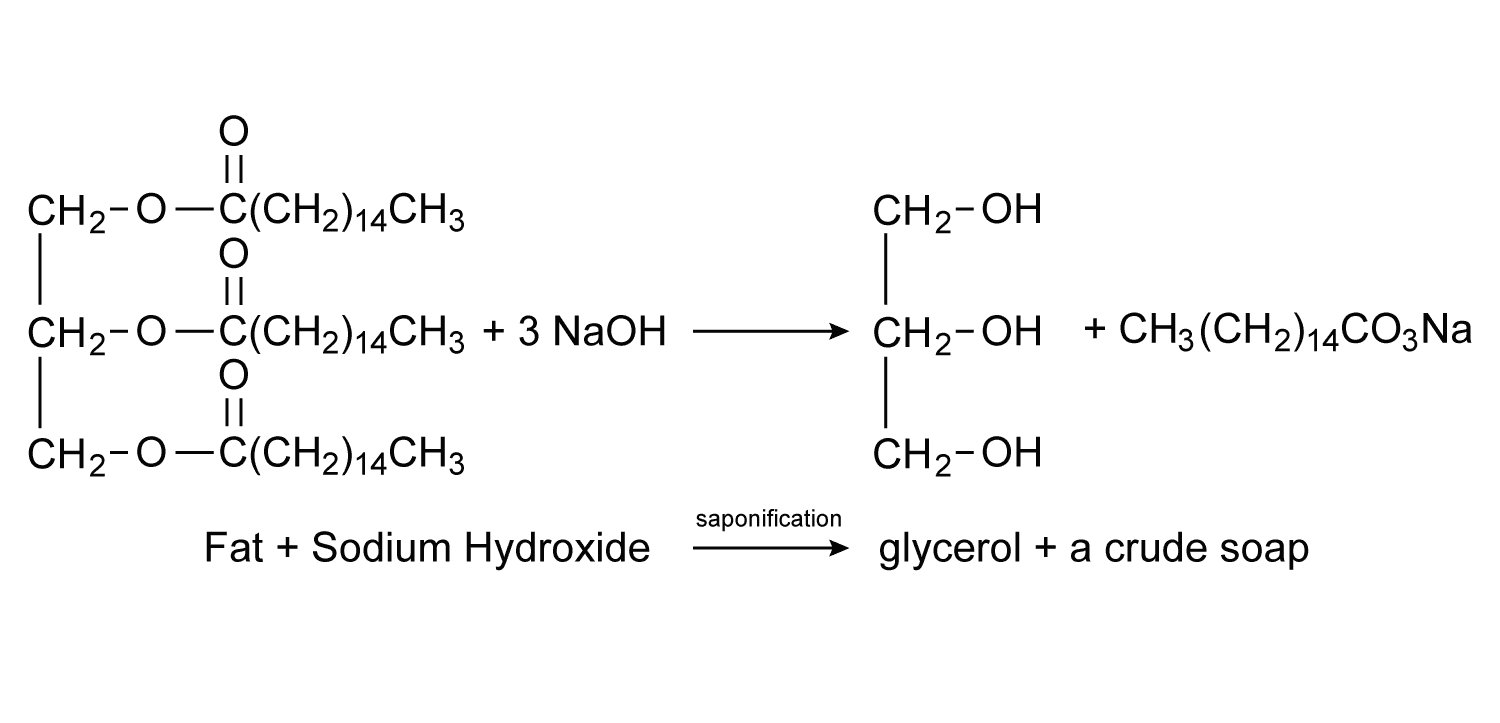
Toilet soaps as they are known are produced by using either Sodium Hydroxide (NaOH) or Potassium Hydroxide (KOH) as the alkaline. When sodium hydroxide is combined with a thick fat such as lard or tallow the result will be a hard soap while potassium hydroxide and a light oil, such as olive oil, will produce a softer or even a liquid soap. When Lithium Hydroxide is used as the alkaline the result is lithium stearate a common industrial lubricant.

So how do soaps perform their job as a cleaning agent? Well, remember that oil and water don’t mix because oils are non-polar molecules while water is a polar molecule. However a soap molecule is a combination of an alkaline and fat. That arrangement produces a molecule that is polar at one end, attracted to water, but non-polar at the other end, attracted to fats and oils.
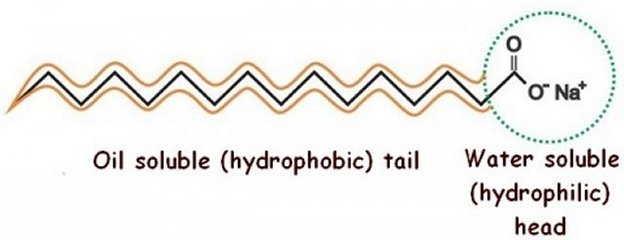
Because of that when used in combination with water soap acts as a surfactant, a material that breaks the surface tension of water allowing the water to more easily dissolve dirt and grime, along with such polar molecules as proteins and sugars, so that they can be washed away.
Soap’s greatest trick however is its ability to encase droplets of oil or fat in tiny spheres of soap molecules called micelles. Unlike oils and fats that do not dissolve in water, these micelles do dissolve allowing the oils and fats to be washed away with the dirt and grime. In other words not only does soap help water to better dissolve the substances it usually can, it also enables water to dissolve substances it generally can’t.
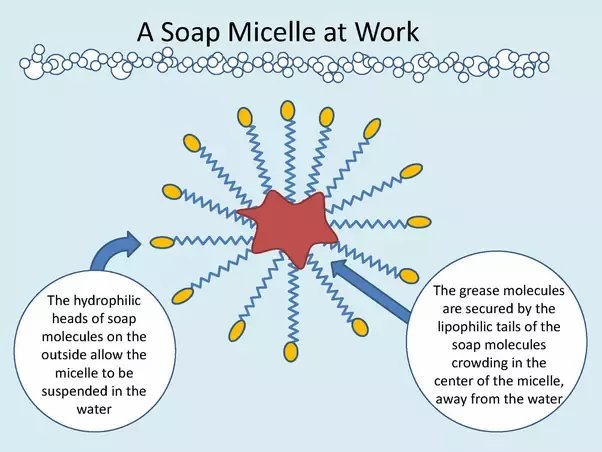
This also makes soap an effective anti-biotic because the harsh alkaline at one end of the soap molecule can break up the protein shells that protect viruses. At the same time the micelles can absorb the fats in the cell walls of bacteria, killing them. Of course modern, manufactured soaps often have various chemicals added to them in order to make them even more ‘anti-bacterial’ but it is worth remembering that any soap can be used as a disinfectant.

Archaeological evidence for the manufacture of soap dates all the way back to ancient Babylon with a cuneiform tablet dated to 2200 BCE that describes the earliest known recipe for soap making. The Egyptians, Greeks and Hebrews all had their own varieties of soaps, mostly produced with olive oil and potash, an alkaline solution made from the ashes of a fire, along with a bit of quicklime. This method of soap making produced a strong and particularly harsh soap.


Surprisingly the Romans, who are well known for their baths, did not care very much for soap. They preferred to clean their bodies by rubbing them with olive oil and then scrapping the oil off with a dull knife called a strigil. They considered the harsh types of soaps made in the eastern Mediterranean as harmful for the skin. Only after becoming familiar with the milder soaps of the Celts and Germans did the Romans start using soap. (Imagine that, the fierce northern barbarians had the gentler soap!)

Both medieval Europe and the Islamic world had soaps but these soaps were generally harsh with an unpleasant smell and so expensive that only the very rich could afford to bathe frequently. Large-scale manufacture of soaps only began in the late 18th century and coincided with a campaign that linked daily washing with good hygiene, ‘cleanliness is next to Godliness!’

Today of course there is a tremendous variety of different kinds of soap available in your local supermarket. There are soaps that can remove the toughest dirt and grime, soaps that actually soften the skin and even soaps that are 9944/100 % pure soap. There are solid bar soaps and liquid soaps, advertised as ‘body wash’, there are even powered soaps. Whatever kind of soap you prefer we all know that regularly washing your hands with soap and warm water is our first line of defense against Covid-19. So wash up and remember, ‘I’m pulling for ya, we’re all in this together’!
