All of the infectious diseases that afflict us have one problem in common that they must solve, how to pass from an infected individual to an uninfected one. Some take the shortest path and move from person to person by touch. Our skin is actually pretty tough however so without a cut or other wound allowing the germs to get inside infection rarely takes place. Some microbes get themselves sprayed into the air by a cough or sneeze in order to be breathed in by their victim, but that’s very much hit or miss with most of the germs left hanging out in space.

Then there are some that actually use another living creature to literally take them from a sick person to new victims. A couple of well known examples of this type of disease are malaria, which uses the mosquito Anopheles gambiae to transmit the bacteria and bubonic plague which uses fleas that are themselves transported by rats. These organisms that pass on diseases from one person to another are referred to a ‘vector’ by epidemiologists and controlling the spread of the vectors, the rats and mosquitoes has often proved to be the most effective way to fight the diseases they spread.

Now some biologists are actually trying to use the technology of gene editing to modify the DNA of the vector organisms in an effort to make those species unable to spread the diseases! See my posts of 5Aug17, 1Sept18, 1Dec18 and 12Jan19 for information on gene editing. Two such projects are now reaching the stage where field-testing could soon begin!
I mentioned the disease malaria above, a disease that most epidemiologists believe is responsible for the death of half of all the human beings who have ever lived. Think of that, half of all the people who have ever died were killed by the single disease malaria. Even today malaria is a terrible scourge in many parts of the world killing an estimated million people yearly while 300-400 million suffer from the disease.
It’s easy to understand therefore that many efforts are underway to fight this deadly disease. One of these efforts involves the use of gene editing techniques to control the population of the Anopheles gambiae mosquito that transports the malaria germ.
Using the gene-editing tool CRISPR a high security lab in Terni Italy has produced a modified form of female mosquito whose mouth parts resemble those of the males. You see it’s only the female mosquitoes who actually bite, sucking the blood of warm blooded animals in which they incubate their eggs. Females with male mouth parts would be unable to bite, unable to breed in the wild.

The idea is to artificially breed large numbers of male mosquitoes with the modified gene and release them into the wild. There they will breed and all of their female offspring will be sterile while the male offspring will continue to propagate the edited gene. It is hoped that this will cause the local population to crash, reducing the occurrence of malaria.
The problem, as it always is with gene editing, is unintended consequences. Both genetics and ecology are extremely complex matters that we still know very little about and releasing GMO mosquitoes into the wild will almost certainly alter the environment in unexpected ways. If nothing less, the crash of the Anopheles gambiae population in an area could lead to another species of mosquito, possibly carrying a different disease such as yellow fever to move in to the empty ecological niche.

The scientists at Terni, led by lab director Ruth Mueller, are aware of the possible dangers, that’s why they’re doing the experiments in a high security lab in order to make certain none of the GMO mosquitoes escape prematurely. To further reduce the danger the mosquitoes will be subjected to long term studies prior to any actual release.
That means that there’s a long way to go and a lot of work still to go on this project but any advance that helps in the fight against malaria would represent a tremendous victory.
The second project I’ll discuss involves combating Lyme disease by modifying the DNA of mice. You see the spread of Lyme disease involves a complex back and forth between mice, fleas and deer; it’s really a disease of deer more than it is of humans. Both the mice and fleas are born uninfected but once a mouse is bitten by an infected flea it becomes a carrier and any fleas that subsequently bite the mouse also becomes carriers, and can then pass the disease to a deer, or a human.
Some mice however appear to be immune to Lyme disease and according to MIT evolutionary biologist Kevin Esvelt that presents an opportunity. Dr. Esvelt has been experimenting with the mice, identifying the genes that provide the immunity. Dr. Esvelt now plans to use the gene-editing tool CRISPR to insert the immunity genes into the reproductive cells of the mice so that all of their offspring are born immune to Lyme disease.

For field testing Dr. Esvelt has proposed releasing thousands of GMO mice onto some of the uninhabited islands off of Nantucket Island in the State of Massachusetts. It happens that Lyme disease is an epidemic on Nantucket; over 40% of the human population there has been infected, so the people of the island are more than ready to at least listen to Dr. Esvelt’s plan.
Now Dr. Esvelt is well aware of the possibility of unintended consequences, genetics and ecology are both very complex subjects after all. That’s what makes trying the plan on a small, uninhabited island first so attractive; the GMO mice will be confined until all of the ramifications have been studied. Only then, when not only Dr. Esvelt but all of the people of Nantucket are satisfied will the experiment move on to phase 2, releasing the mice onto Nantucket itself.
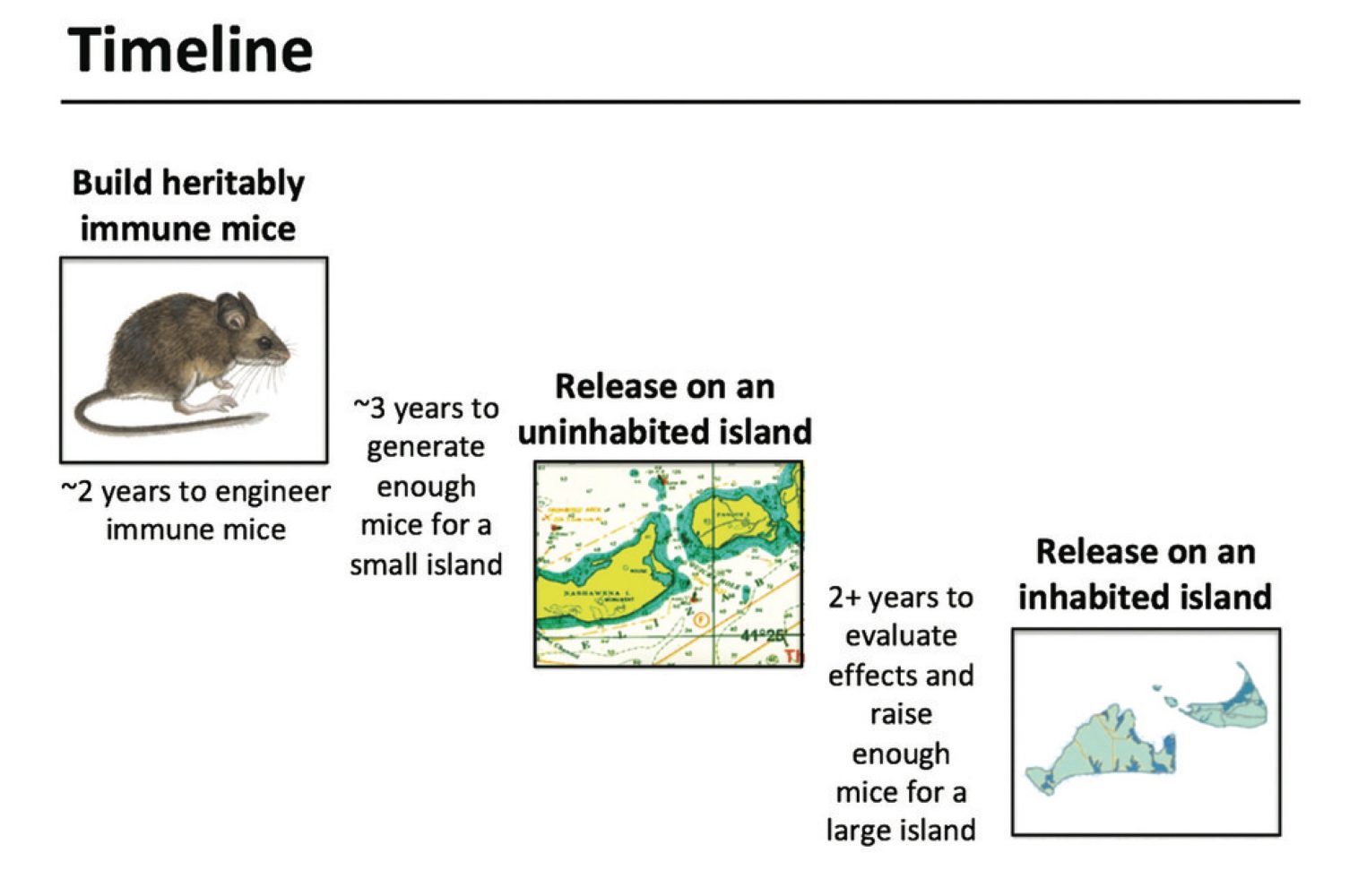
In other words this is also a project that will take years before it can be called a success. Still, if the genetically modified mice do help to eradicate Lyme disease from the islands of Massachusetts then we will have another potent weapon in our fight against infections like Lyme disease.
