Remember back in your high school science class how, when your teacher was talking about the periodic table of the elements he told you that Uranium, element number 92, was the heaviest ‘naturally occurring’ element. I bet he then went on to say that all of the elements with a higher atomic number had been artificially created in ‘atom smashers’.
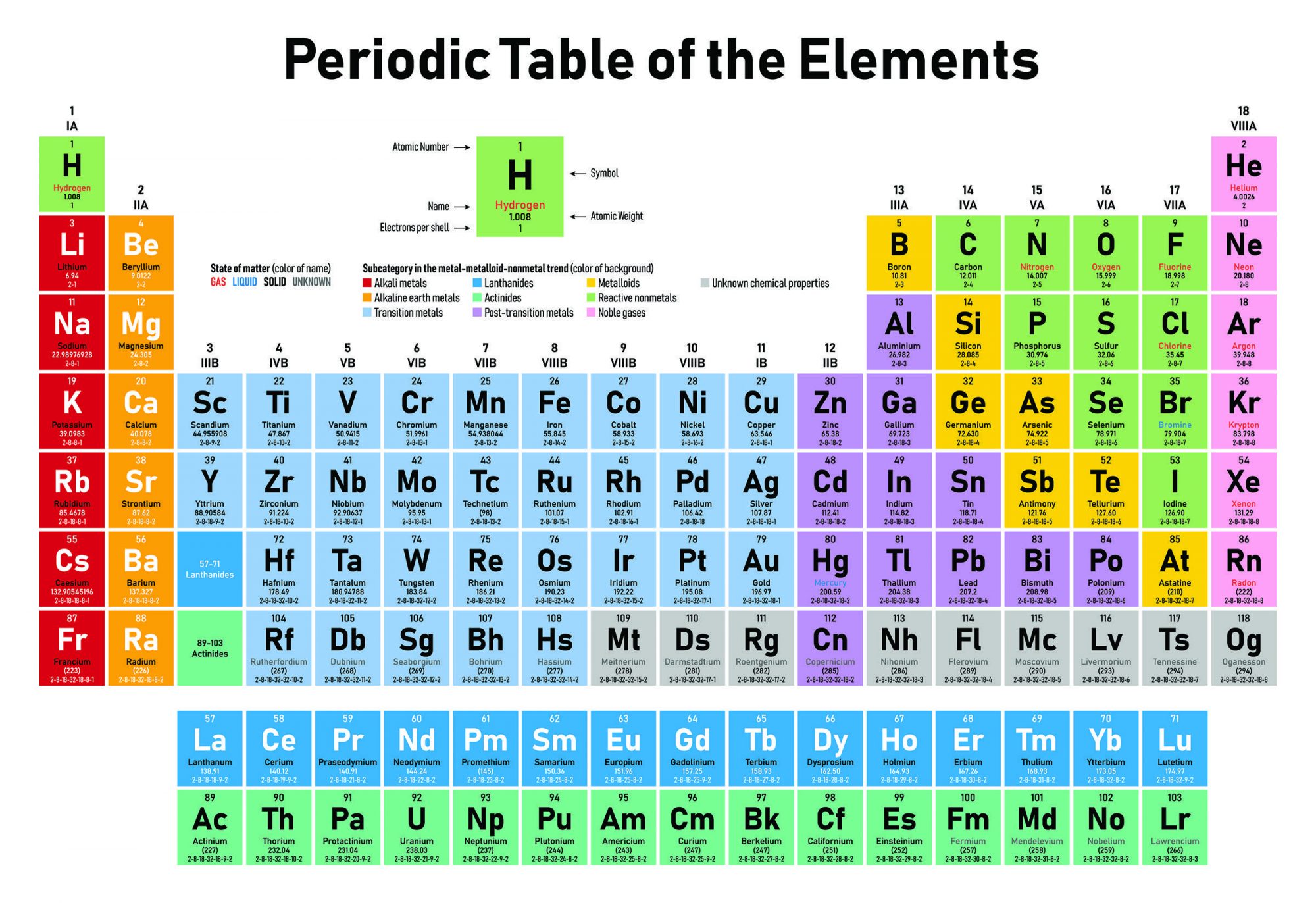
You may also remember that the atomic number of an atom is simply the number of protons in its nucleus. Uranium has 92 protons so it’s element 92 while carbon on the other hand has 6 protons so it is element number 6. Atoms with the same number of protons have the same chemical properties, which is why we say they are the same chemical element.

The atomic mass of an atom however is the sum of the number of protons plus neutrons in the nucleus. Two atoms can have different numbers of neutrons but still be the same element so long as they have the same number of protons. Atoms with the same number of protons but different numbers of neutrons are called isotopes of the same element. For example carbon 12 has 6 protons, which makes it carbon, along with 6 neutrons while carbon 14 has 6 protons, still carbon, but 8 neutrons. The extra two neutrons make carbon 14 unstable, radioactive, which makes it useful for radiocarbon dating.

Uranium has no stable isotopes, they are all radioactive. Its most stable isotope is U 238 with 92 protons, 146 neutrons and a half-life of about 4.5 billion years, which means that the Earth today has just about half the amount of U 238 it had when the solar system first formed. Elements with higher atomic numbers have much shorter half-lives, the most stable isotope of Plutonium, element number 94, is P 244 with a half-life of 80 million years. Which is why whatever Plutonium the Earth started with 4.3 billion years ago has all decayed away.

Scientists have been manufacturing ‘Trans-Uranium’ atoms since just a few years after they realized that atomic nuclei were composed of protons and neutrons. Neptunium, number 93, and Plutonium were both first manufactured in the year 1940 at what is now known as Lawrence Berkeley Labouratory by bombarding atoms of Uranium with neutrons. Over the next 34 years another twelve new elements, right up to Seaborgium, number 106, would be developed at the California labouratory.

In the late 1970s a new labouratory, the Gesellschaft für Schwerionenforschung (GSI) or the Society for Heavy Ion Research in Hessen Germany first began its studies. This team would go on to be the first to manufacture elements 107 to 112 between the years 1980 and 1996. By this time the technique of simply taking the heaviest element yet produced and bombarding it with protons or neutrons was no longer working. You see as the atomic number gets higher the nucleus quickly becomes even more unstable so that the atoms that were produced only lasted for seconds making it virtually impossible to push them up one stage higher before they decayed.

Instead a technique known as ‘cold fusion’ was developed. Cold fusion by the way has nothing to do with the erroneously announced ‘cold fusion’ of hydrogen into helium back in the 1980s. The technique of cold fusion involves slamming a middling sized nucleus into a heavy but fairly stable nucleus. One example is slamming a Neon nucleus into Uranium to produce Nobelium, element 102.
Cold fusion is a very delicate technique because you have to use just the right amount of energy. Too little and the electrostatic repulsion of the protons in the two nuclei will keep them from ever touching. Too much and the collision will just obliterate both nuclei.
Throughout this entire period there was also a Russian labouratory that was devoted to the study of trans-uranium elements called the Joint Institute for Nuclear Research (JINR). Despite having played an important role in nuclear research for many years, the technique of cold fusion was developed at JINR for example; the Russians had never succeeded in being the undisputed first to develop a new element. That situation lasted until 1999 when JINR became the first labouratory to demonstrate the existence of element Flerovium, element 114.

JINR went to produce the next 4 elements with the latest element yet being number 118 in 2010, the element was named Oganesson after Yuri Oganessian the head researcher at JINR. In the ten years since Oganesson research has hit a brick wall as the cold fusion technique has proven unable to produce enough nuclei, that last long enough to be observed sufficiently enough that a new element can be announced.

But JINR is currently gearing up for a new attempt. A new atom smasher known as the Superheavy Element Factory (SHEF) has been assembled and once a new supply of the element Californium arrives to be used as a target the testing will commence. The Californium itself has to be manufactured at Lawrence Berkeley Labouratory and with a half-life of only approximately 500 years it is both dangerously radioactive and difficult to produce and handle.

The new goal at JINR is actually not element 119 but element 120 because calculations indicate that 120 could be a island of stability, lasting perhaps hundreds, possibly even thousands of times longer than elements that are slightly smaller. This stability arises from the laws of quantum mechanics where certain magic numbers of identical particles can arrange themselves in orbitals that produce a degree of permanence. Testing at the SHEF is slated to commence this spring so it’s possible that we may know if the theory is correct before the year is out.

So how far can we go, that’s almost impossible to say. With each step higher it not only becomes harder to produce atoms of new elements but harder still to detect them. Still physicists are clever creatures and they’ve always found a way to surmount whatever difficulty arises.
There is a theoretical calculation, again based in quantum mechanics, which indicates that element 172 might be an impassible brick wall. Any more protons in a nucleus and they will start grabbing electrons to fuse into neutrons until the number of protons is reduced back down to 172. Of course that obstacle, if real is many years away and there’s still more than 50 elements to be manufactured before we reach it.
So the nuclear physicists will keep on working. If element 120 does turn out to be an island of stability you can bet that it won’t be long before labouratories are using it as springboard to even higher elements. The science of Trans-Uranium elements has not only taught us a great deal about how atoms are composed but at the same time advanced techniques for high-precision, high-sensitivity sensors as well as data collection and analysis. So the periodic table of the elements has grown quite a bit since I first studied it in high school, and I hope that I do live long enough to see a few more elements added to it.
